![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
69 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
The atmosphere
|
Is a mixture of gases surrounding the earth. Human activity has added other gasses to the atmosphere which are called pollutants.
|
|
|
|
Gases in the atmosphere
|
Nitrogen 78%
Oxygen 21% Argon and other 1% Carbon dioxide 0.04% Water vapour different concentration across the world |
|
|
|
Pollutants in the air
|
Burning fuels produces carbon monoxide, carbon dioxide, nitrogen oxides and sulphur dioxide.
Incomplete combustion of fuels releases particulates. |
|
|
|
Effects of pollutants
|
Carbon monoxide is toxic, causing blood poisoning.
Sulfur dioxide causes acid rain. Carbon dioxide causes greenhouse effect. Particulates dirtys buildings and affects lungs. |
|
|
|
Story of the atmosphere
|
The earths atmosphere used to be co2 and water vapour, from volcanoes. As the earth cooled the water condensed forming the oceans. Carbon dioxide dissolved forming sedimentary rocks. Some carbon was trapped underground forming fossil fuels.
|
|
|
|
Evidence of earths atmosphere
|
Air bubbles in the ice cores and fossils. Fossils suggest that many organisms underwent photosynthesis using co2 to form oxygen.
|
|
|
|
Reactants
|
The substance that react together are called reactants.
|
|
|
|
Products
|
The substance formed during a reaction.
|
|
|
|
Conservation of atoms
|
No atoms are added or taken away.
|
|
|
|
Combustion
|
When a chemical reacts rapidly with oxygen, releasing energy.
|
|
|
|
Hydrocarbons
|
Petrol, diesel fuels and fuel oils. Their molecules are made up of carbon and hydrogen atoms, combing with oxygen atoms to produce carbon dioxide and water vapour.
|
|
|
|
Incomplete combustion
|
Forms particulates. Also if there is not enough oxygen present to burn the the fuels completely. Carbon monoxide a toxic gas is formed.
|
|
|
|
Particulates
|
Very small pieces of solid mainly carbon, made during incomplete combustion
|
|
|
|
Sulphur dioxide
|
Formed when fuels containing sulphur compounds are burn. It then in turns reacts with water and oxygen in the air producing acid rain. Corroding buildings and kills plants.
|
|
|
|
Reducing carbon dioxide in the air
|
Burn less fossil fuels, although we heavily depend on them we would need alternative energy sources
|
|
|
|
Reduce a person's use of electricity
|
Turn down the heating
Insulate people's houses Turn off lights Use low energy lamps Don't leave TV's on standby |
|
|
|
Reduce the amount of sulphur dioxide is given off from power stations
|
Remove sulphur dioxide from natural gas and fuels
Wet scrubbing |
|
|
|
Wet scrubbing
|
Removing sulphur dioxide from flue gases in the chimney. By either spraying the flue gases with seawater droplets or using powdered lime (calcium oxide) and Water. The sulphur dioxide reacts and forms calcium sulphate which is a solid and can be collected and removed.
|
|
|
|
Electromagnetic static precipitator
|
Pass the particulates (carbon and gas) through an electrostatic precipitator. The particulates pick up a negative charge so are attached to the positive charged plate and collected and disposed of.
|
|
|
|
Reducing exhaust gas from motor vehicles
|
Burning less fuel with more efficient engines
Low sulphur fuels Catalytic converters Public transport Legal limits in exhaust emmisions |
|
|
|
Catalytic converters
|
Convert nitrogen monoxide to nitrogen and oxygen and carbon monoxide to carbon dioxide
|
|
|
|
Alternative fuels for cars table - benefits and posotives
|
Biofuels and electricity.
|
|
|
|
Oxidation
|
Oxidation reactions add oxygen to a chemical.
|
|
|
|
Mixture
|
Two or more different chemicals mixed but not chemicals joined together.
|
|
|
|
Crude oil
|
Crude oil is a raw material obtained from the earths crust. It is a mixture of different chemicals most are hydrocarbons. There are chains of molecules of varying length made from hydrogen and carbon atoms.
|
|
|
|
Crude oil refinery
|
Separating the hydrocarbons into fractions using fractional distillation. Each have different boiling pointspoints. Heated around 400°C. The smallest molecules have the lowest boiling points so move to top of tower.
|
|
|
|
Fractional distillation diagram.
|
The molecules condense at each level depending on their boiling point separating them.
|
|
|
|
Corporation between boiling point for hydrocarbons
|
The boiling point of hydrocarbons increase as the number of carbon atoms in the molecules increases
|
|
|
|
Polymerisation
|
Some small molecules (monomers) can join together to make very long molecules called polymers. This process is called polymerisation. Many polymers are made from chemicals that are obtained from crude oil.
|
|
|
|
Arrangement of particles
|
Stronger forces between the particles of a solid structure, the more energy needed to break it and higher melting point.
|
|
|
|
List of modifying polymers
|
Polymer chains lengths
Plasticisers Cross links Increasing cyrstalinity lining up chains |
|
|
|
Changing length of polymer chains
|
Long polymer chains have stronger forces of attraction than shorter ones. By lengthening the chain it make it stronger and less flexible.
|
|
|
|
Adding plasticisers
|
Plasticisers are small molecules that can be added to polymers during their manufacturer. Separating the polymer molecules slightly weakening the force. Making it softer and more flexible.
|
|
|
|
PVC and uPVC properties
|
uPVC is unplasticed is hard. While PVC is plasticised is soft.
|
|
|
|
Cross links
|
Chemical bonds can be formed to link together the chains of some polymers. These cross links make the material tougher and less flexible.
|
|
|
|
LDPE low density polythenes
|
Some polymer chains have beaches, so not lined up regularly, not crystalline. Low density, weaker forces, weaker material, low melting point
|
|
|
|
HDPE high density polythenes
|
Does not have side branches, line up regularly, crystalline structure. High density, strong forces, strong material, high meting point.
|
|
|
|
Cyrstaline polymer
|
A polymer with molecules lined up in a regular way as in a crystal.
|
|
|
|
Nanotechnology
|
The use and control of tiny matter called nanoparticles. Measured in nanometer (nm).
|
|
|
|
Use of nanoparticles
|
Sports equipment - to make them lighter but stronger
Medical dressings and socks - nanoparticles stops clothes from absorbing smell of sweat because of antibacterial properties. |
|
|
|
Nanoparticles harmful effects
|
They may be able to enter our brain from the blood stream and cause harm.
|
|
|
|
Magnetic data in the tectonic plates
|
As the magma solidifies it becomes magnetised in the direction of the earths magnetic field at the time.
|
|
|
|
How does sedimentary rocks provide evidence?
|
Fossils telling us about the animals and what the environment was like at the time.
Grains of sand can be identified as desert or beaches Ripples in the rock tell us if it was wind or water |
|
|
|
Formation of coal
|
When a plant has died it formed peat due to lack of oxygen. The peat was the buried under sedimentary rocks, them due to compression and heat is forms coal.
|
|
|
|
Salt marshes formation
|
Seawater which was inland millions of years ago formed salt marshes, because as the water evaporated it left deposited if salt.
|
|
|
|
Salt and main uses of salt
|
Sodium chloride. Food industry as a preservative and flavouring. Also icy roads as salt water has a lower freezing point.
|
What elements is it
|
|
|
Obtaining salt - two methods
|
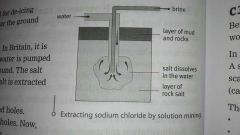
From the sea or from underground
Mining - but it had impurities such as clay can be used for roads. Solution in water - water is forced down a bore hole into rock. The salt dissolves making a brine. The salt crystallises and then is filtered or centrifuge. |
|
|
|
Centrifuge
|
In tubes fill with water with salt in suspension, spin it fast so the sentripital force causes the salt crystals to go to the sides.
|
|
|
|
Environmental impacts
|
Extraction of salt can cause large underground caverns. Leading to collapsing of the ground, causing damage to houses and water works. To solve space the holes so the surface is supported.
|
|
|
|
Risk of salt to humans
|
Humans need sodium in their diet. But eating too much salt raises the blood pressure and having a stroke. Governments have regulations on how much salt.
|
|
|
|
Alkaline what pH and use of them before 1700's
|
Higher than pH7.
Converting oils and fats in salt Making glass Neutralising acidic soils Dyeing clothes |
|
|
|
What does an acid + alkaline make
|
Acid + alkaline - - - > salt + water
|
|
|
|
What does an alkaline hydroxide + acid make
|
Alkaline hydroxide + acid - - - > salt + water
|
|
|
|
Examples of alkalines
|
Solvable hydroxide and carbonates
|
|
|
|
What does a alkaline carbonate + acid make
|
Alkaline carbonate + acid - - - > salt + water + carbon dioxide
|
|
|
|
LeBlanc process
|
Making sodium chloride. Mix sulphuric acid with sodium chloride heating it with charcoal and limestone.
|
|
|
|
Pollutants made during the LeBlanc process
|
Acid gas (hydrogen chloride). Toxic solid waste that is dumped but let's of toxic gas (hydrogen sulphide).
|
|
|
|
Solving LeBlanc process problems
|
dissolve hydrogen chloride gas in water making hydrochloric acid.
|
|
|
|
Waterborne diseases
|
Typhoid, cholera and dysentery
|
|
|
|
Treatment of water
|
Treat water with chlorine, kills micro-organisms. But drinking chlorine treated water some disinfectant byproducts called torholmethanes can form.
|
|
|
|
Brine solution elements and symbols
|
Sodium chloride (NaCl) + water (H2O)
|
|
|
|
Electrolysis of brine and diagram
|
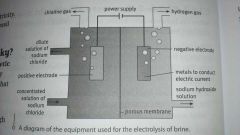
The process of using an electrical current to bring about a chemical change. Manufacture chlorine from salt. Also to obtain sodium hydroxide and hydrogen.
|
|
|
|
Electrolysis of brine product uses
|
Chlorine - disinfectant, plastic production, hydrochloric acid
Sodium hydroxide - remove pollutants in water, paper making Hydrogen - hydrochloric acid, pollutant fuel |
|
|
|
Environmental issues of electrolysis
|
Use of electricity which is made from buying fossil fuels
Mercury is commonly used which is toxic. |
|
|
|
What is a persistent organic pollutant (POP)
|
It is a class of chemical that takes a long time to break down and can harm people and the environment. Toxins then accumulate in fatty tissues of animals. Can lead to death.
|
|
|
|
Polyvinyl chloride (PVC)
|
Is a plastic containing carbon, hydrogen and chlorine. Produced in the process polymerisation.
|
|
|
|
Life cycle assessment (LCA)
|
Assess the environmental impact of manufacture and use of different materials and products.
|
|
|
|
Key features of of a life cycle assessment
|
Requirements for energy input
Environmental impact of making product from the raw material, using product, disposing of product (landfill/recycling) |
|
|
|
Henry deacon making chlorine
|
Hydrogen chloride + oxygen - - > chlorine + water
Chlorine is used as bleach to whiten paper and textile. Also to kill microoramisms in water. |
|

