![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
81 Cards in this Set
- Front
- Back
|
What type of rock is limestone? What is it made of? What is the symbol equation? |
Sedimentary Calcium carbonate CaCO3 |
|
|
What can limestone be used for? |
Building materials and making cement mortar and concrete |
|
|
What is the problem with using limestone as a building material? |
Limestone can be eroded by acid rain but this is a slow process |
|
|
What happens when limestone is heated? What scientific process is this? |
It decomposes by thermal decomposition |
|
|
What is made when calcium oxide reacts with water? |
Calcium hydroxide |
|
|
What is calcium hydroxide used for? |
Neutralises soils and lakes, preventing crop failure |
|
|
What is the symbol equation for calcium oxide? |
CaO |
|
|
What products does calcium carbonate break down into when heated? |
Calcium oxide and carbon dioxide |
|
|
What is the symbol equation for calcium hydroxide? |
Ca(OH)2 |
|
|
What is made when calcium hydroxide reacts with carbon dioxide? |
Calcium carbonate |
|
|
How is cement made? |
When limestone and clay is roasted in a rotary kiln |
|
|
How is mortar made? |
When cement, sand and water are mixed together |
|
|
How is concrete made? |
When mortar, aggregate, sand and water are mixed |
|
|
What is a metal ore? |
A mineral that contains enough metal to make it economically viable to extract |
|
|
What does the method of metal extraction depend on? |
How reactive the metal is |
|
|
How can unreactive metals be obtained? |
Through panning |
|
|
How can metals that are less reactive than carbon be obtained? |
It can be heated with carbon |
|
|
What form are most metals found? |
As metal oxides |
|
|
How can metals that are more reactive than carbon be obtained? |
Electrolysis |
|
|
How is copper extracted? |
Heating ores in a furnace, known as smelting. The copper is then purified by electrolysis |
|
|
Which electrode do the copper ions go to? |
The cathode because they are positively charged •Cathode •Anode Is Is Positive Negative |
|
|
What new methods are being used to extract copper due to a shortage of copper-rich ores? |
Phytomining, bioleaching of low grade copper ores |
|
|
What is phytomining? |
Uses plants to absorb copper, as the plant grows they absorb and store copper by removing it from the soil. Plants are then burned and the ash contains copper in relatively high quantities |
|
|
What is bioleaching? |
Uses bacteria to extract metals from low-grade ores. A solution containing bacteria is mixed with low-grade ore. The bacteria converts the copper into a solution where it can be easily extracted |
|
|
What is the name of the copper solution that is created during bioleaching? |
Leachate |
|
|
How can iron be produced? |
By reducing iron oxide in a blast furnace |
|
|
How is steel made? |
When carbon is added to iron |
|
|
What is wrong with the iron obtained from a blast furnace? |
It contains impurities which makes it very brittle so it has limited uses at this stage The impurities must be removed to produce the useful pure iron for steel |
|
|
What are the properties of steel with a high carbon content?What are the properties of steel with a low carbon content? |
Hard and strong Soft and malleable |
|
|
What are the advantages of stainless steel? |
Hard Resistant to corrosion |
|
|
What are the properties of the transition metals? |
They are good conductors of heat and electricity , hard and mechanically strong, they have high melting point (except mercury), and are malleable |
|
|
How are aluminium and titanium extracted? |
Electrolysis |
|
|
What is copper useful for? |
Wires (good conductor of electricity and ductile) and Pipes (unreactive and malleable) |
|
|
What is aluminium used for? |
Drinks cans - Light weight, lightweight vehicles - Resistant to corrosion, window frames - Low density and aeroplanes |
|
|
What is titanium used for? |
Jet engines -Low density, nuclear reactors - Resist corrosion, replacement hip joints |
|
|
Why is it important to recycle metals? |
Saves money and energy, conserves natural resources and reduces global warming |
|
|
What is crude oil made of? |
A mixture of hydrocarbons |
|
|
What type of hydrocarbons are present in crude oil? |
Alkanes |
|
|
What is the general formula for an alkane? |
CnH2n+2 The number of hydrogen atoms is double the amount of carbon atoms and 2 extra Eg. C2H6 |
|
|
What is the formula for methane? |
CH4 |
|
|
What is the formula for ethane? |
C2H6 |
|
|
What is the formula for propane? |
C3H8 |
|
|
What is the formula for butane? |
C4H10 |
|
|
Why are alkanes saturated?What property does this result in? |
Because each atom is joined together to other atoms by a single bond Relatively unreactive apart from combustion |
|
|
What happens during fractional distillation? |
Crude oil is evaporated and its vapours condense at different temperatures, separating them off through the fractions E.g. Petrol, butane etc. |
|
|
What will each fraction contain? |
Hydrocarbons with a similar number of carbon atoms so crude oil is not completely separated |
|
|
What are the properties of the small hydrocarbons? (Fraction exits at the top of the column) |
Low boiling points and is very volatile (how easy it turns into a gas), it flows easily and ignites easily |
|
|
What are the properties of the large hydrocarbons?(Fraction exits at the bottom of the column) |
High boiling points, it's not very volatile (how easy it turns into a gas), it does does not flow easily, does not ignite easily |
|
|
What temperature is the bottom of the column? |
350 degrees |
|
|
What temperature is the top of the column? |
25 degrees |
|
|
From the top of the tower to the bottom, what is the order of the fractions and what are the products uses? |
•LPG (Liquefied petroleum gas) - used as fuel. •Gasoline/Petrol - Fuel for cars •Naphtha-Making chemicals •Kerosene - Aircraft fuel Diesel - Fuel for cars, lorries, buses etc •Oil - heating oil, lubricating oil, fuel oil etc •Bitumen - Road surfaces |
|
|
What are the advantages of using hydrogen as a biofuel? |
Water is the only product of combustion, making it a clean fuel Water can be used to make hydrogen |
|
|
What are the disadvantages of using hydrogen as a biofuel? |
•Currently no low energy ways to extract hydrogen from water •Hydrogen is a gas therefore it's difficult to store in large quantities • Hydrogen is flammable so there are considerable safety issues |
|
|
What are the advantages of using ethanol as a biofuel? |
•Renewable source of energy so therefore preserves fossil fuels •Sugar beet is what is used to produce ethanol, a plant that grows rapidly in hot climates •Sugar beet aborts carbon dioxide from the atmosphere and therefore decreases the rate of global warming |
|
|
What are the disadvantages of using ethanol as a biofuel? |
•Sugar beet can only be grown in a hot country •Carbon dioxide is a produce of combustion |
|
|
What are biofuels? |
Fuels that are produced from plant matter such as sugar |
|
|
Why are alkanes saturated? |
Because each carbon atom has as many bonds formed with hydrogen atoms as possible due to the single bond |
|
|
What process occurs when you break down long hydrocarbon chains into smaller ones? |
Cracking |
|
|
Why do we break down long hydrocarbon chains? |
Because they are more useful for things such as fuel |
|
|
What does cracking involve? |
Heating the hydrocarbon until it vaporises |
|
|
What sort of reaction is cracking? |
Thermal decomposition |
|
|
What test is used to discover an alkene? |
Bromine water turns colourless if it's mixed with an alkene. Stays orange if it's mixed with an alkane |
|
|
What sort of bond does an alkane have? |
A single bond |
|
|
What sort of bond does an alkene have? |
Double bonds |
|
|
What is the general formula for an alkene? |
CnH2n E.g. C2H4 |
|
|
What are alkenes used for?Why? |
Making polymers Because of the stronger double bonds |
|
|
What is the process of converting alkenes into polymers? |
Polymerisation |
|
|
What are plastics? |
Synthetic polymers |
|
|
What is PVC (Polyvinyl chloride) used for? |
Waterproof items Drainpipes Electrical insulators |
|
|
What is poly(ethene) used for? |
Plastic bags and bottles |
|
|
Why are vegetable oils used in cooking? |
•It has a higher boiling point than water so they can cook food at higher temperature resulting in the food being cooked quicker •Different flavour added •Using vegetable oil increases the energy released by food when consumed |
|
|
What are the characteristics of an emulsion? |
Thicker than oil or water Better texture Better appearance Better coating ability |
|
|
What are some examples of emulsions? |
•Salad dressings •Ice cream •Cosmetics •Paints |
|
|
What is an emulsifier? |
A substance that helps to stabilise an emulsion, it helps oil and water mix |
|
|
What do emulsifier molecules contain? |
Hydrophilic head that mixes with water molecules Hydrophobic tail that mixes with oil molecules |
|
|
What is the core of the earth made of? |
Nickel and iron |
|
|
What did Alfred Wegener propose? |
South America and Africa were once a single land mass. Proposed the movement of the crust was responsible for the separation of the land - Continental drift which is the tectonic theory |
|
|
What causes convection currents? |
Intense heat released due to radioactive decay deep in the Earth |
|
|
What is the Earth's lithosphere? |
Crust and upper part of the mantle |
|
|
What was the Earth's atmosphere like 4 billion years ago?Why is this? |
Majority of it was carbon dioxide Small proportions of methane and ammonia Water vapour which condenses to form oceans Intense volcanic activity |
|
|
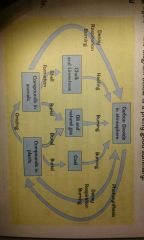
Describe the carbon cycle |
|

