![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
draw the picture of what would happen if a cell used glucose?
|
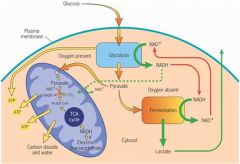
p
|
|
|
|
why is oxidation of hydrocarbons so lucrative?
|
1)oxidation of hydrocarbons to reduce oxygen and form h20 to form water releases a lot of free energy: -56 kcal
2) if cellular metabolism can strip H atoms from hydrocarbons and combine them with oxygen, a lot of energy can be generated that can do useful work: drive ion gradients, make atp |
|
|
|
during glycolysis and the krebs cycle, what happens to H atoms?
|
-they are stripped away from carbohydrates
-next, they are transferred to NAD+ and FADH to make NADH and FADH2 -the equation then proceeds as follows NADH + ½ O2 + H+ <> H2O+ NAD+ delta g = -52 kcal/mole |
|
|
|
is the oxidation of NADH from oxygen practical?
|
no because it would require a lot of energy
|
|
|
|
is the oxidation of NADH coupled to atp synthesis?
|
it couples it inderectly by allowing small packets of energy to be released at a time
|
|
|
|
does oxygen have a high or low electron affinity?
|
high
|
|
|
|
E’o is a measure of
|
ΔG’o for the reaction
|
|
|
|
ΔG’o =
|
- nFE’o
n=transfer of electrons |
|
|
|
a very high electron affinity indicates what?
give one example |
a very positive “standard redox potential”, E’o - +0.816 V
|
|
|
|
Clearly if E’o is very positive
|
the molecule will have a high electron affinity and will want to accept electrons
|
|
|
|
why is it bad for NADH to be directly oxidized by oxogyn?
what happens instead? |
oxygen has a very high electron affinity and will release too much energy
instead, NADH is oxidized by a molecule with a much lower electron affinity (close to NADH): coenzyme Q (aka. Ubiquinone, UQ or Q)...coenzyme q is the first in a chain of electron carriers this event is catalyzed by NADH dehydrogenase the process up to oxygen is sort of like a ladder: electrons are transferred to molecules with higher and higher electron affinities until they reach oxygen each time electrons are transferred they release energy |
|
|
|
NADH dehydrogenase
|
complex 1
an integral membrane protein found in the mitochondrial matrix catalyzes the transfer of electrons from NADH and coenzyme Q |
|
|
|
describe the carriers of the electron transport chain?
|
at least 5 types
The carriers of the electron transport chain are of five types (at least) – most are small molecules or atoms imbedded in prosthetic groups within proteins Flavoproteins – a protein that binds a prosthetic group FAD or FMN (derived from vitamin B2) Cytochromes – proteins that contain the heme prosthetic group – use the Fe2+/Fe3+ transformation. Three copper atoms within the mitochondrial membrane. Cu2+/Cu+ Ubiquinone – a small molecule that exists in a pool in the lipid bilayer Iron-sulfur protein complexes - Fe and S atoms embedded in protein |
the electron transport chain can go "fuc ic"
|

