![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
82 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
formula for oxidation of glucose
|
C6H12O6>6CO2+6H20
|
|
|
|
what is the purpose of oxidation of glucose?
|
-releases a lot of energy (-686 kcal per mole)
-this energy can be used to make atp ADP + Pi> ATP (+11.5 kcal per mole under cellular conditions) |
|
|
|
overall what happens in glycolysis?
|
one molecule of glucose is converted to two molecules of pyruvate with the net production of 2 ATP and 2 NADH molecules
|
|
|
|
what are the 3 phases of glycolysis?
|
|
|
|
|
where does glycolysis occur?
|
cytosol
|
|
|
|
name 2 energy storage compounds?
|
ATP and NADH
|
|
|
|
how much ATP and NADH is created in glycolysis per molecule of glucose?
|
-4ATP (2 is used up in the process)
-2 NADH |
|
|
|
How is a positive delta G made negative in glycolysis?
|
low [product] due to constant removal
-reactions are coupled |
|
|
|
Is Oxygen needed in glycolysis?
|
no
|
|
|
|
draw the complete glycolysis pathway
|
draw it: 110
|
|
|
|
what would the free energy change graph of glycolysis look like?
|
draw it
|
|
|
|
What does fermentation do?
|
uses NADH to reduce pyruvate, regenerates NAD+
|
|
|
|
draw fermentation reaction for yeast
|
draw it: 113
|
|
|
|
draw the overall glycolysis reaction
|
Glucose + 2ADP + 2Pi + 2NAD+ >2 pyruvate + 2NADH
+ 2ATP + 2H+ + 2H20 |
|
|
|
when organisms have little or no O2, how do they get energy?
|
glycolysis and fermentation
|
|
|
|
membrane function: name and describe each
|
Membrane Functions
|
membrane can select people to respond in earth
|
|
|
Charles Overton
|
1890
postulated the presence of a lipid coat on the cell surface -reported that non-polar substances readily entered cells while polar substances did not |
picture over a ton of lipids on the cell surface
|
|
|
lipophillic
|
non polar
|
|
|
|
E gorter and F Grendel
|
1925 used the Langmuir trough to conclude that the bilayer was 2 units thick.
fig 4.3 on 123 |
G and G are special agents that work for the CIA and have been using incarcaration techniques of seeing how close they can get people.
|
|
|
describe what gorter and grendel concluded: give the entire process
|
|
|
|
|
Hugh Davson and James Danielli
|
recognized that a membrane could not be lipid only because this would not account for all materials crossing it
devised protein/lipid sandwich...hydrophilic proteins are present in one or more layers...on both sides of the lipid bilayer. - |
double D's in membrane
|
|
|
determined lipid bilayer
|
gorter and grendel
using langmuir trough |
|
|
|
determined that a lipid bilayer must have proteins embedded in it?
|
davson and danielli
|
double d's in the membrane look like protein
|
|
|
what is the davson-danielli model
|
draw it
124 |
|
|
|
Singer and Nicholson
|
1972
proposed fluid-mosaic model of membrane |
picture nick singing releasing a lot of fluid
|
|
|
fluid mosaic model of membrane proposed by who?
|
Singer and Nicholson
|
when u sing u bring up fluid and nick is useless except for fluid
|
|
|
fluid mosaic model
|
-present in fluid state (not so in previous structures)
-proteins are embedded discontinously in membrane (prvious structures did not have it this way) -lipids can self assemble into liposomes and sheets singer and nicholson proposed |
sing though your lips, but nick sings through his ass because he never knows what he is talking about
|
|
|
liposome
|
sperical vesical composed of a lipid bilayer
|
|
|
|
what can liposomes be used for?
|
drug delivery
|
|
|
|
common structure of biomembranes
|
thin 6-8 nm double layer of lipid into which protein molecules are embedded
|
|
|
|
biomembrans are held together by what?
|
weak bonds-especially the hydrophobic effect that binds lipid tails together
|
|
|
|
most lipids are free to move within the plane of the membrane...T/F
|
true
|
|
|
|
what are water soluble compounds
|
polar or charged materials
|
|
|
|
what does a lipid bilayer serve to do?
|
-ipermeable barrier to water-soluble compounds
-lipids provide the flexibility, stability, and electrical resistance properties of membranes |
|
|
|
most abundandant type of membrane lipids?
|
phospholipids (phosphoglyderides)....15-50percent of membrane
|
|
|
|
what is the head group in phosphoglycerides?
|
phosphate + polar group
|
|
|
|
what are phosphoglycerides made of?
draw structure....pg126 |
1)diacylglycerol
2)phospate 3) A polar group covalently linked to the phospate head group, glycerol, and a fatty acid |
|
|
|
are the two fatty acid chains in phospolipids usually identical?
|
no
|
|
|
|
how many carbons does a fatty acid chain normally have?
|
16-20
|
|
|
|
what is saturation relating to lipids
|
the number of double bonds
|
|
|
|
what is a double bond in a fat
|
unsaturated and normally the cis type
|
|
|
|
what do double bonds do to lipids?
|
creates a pronounced kink in the otherwise flexible tail because bonds are normally cis type
|
|
|
|
fatty acids that lack double bonds
|
saturated
|
|
|
|
fatty acids possessing double bonds are
|
unsaturated
|
|
|
|
what can affect the packing of a lipid?
126 |
chain length and saturation levels
|
|
|
|
spingolipids
126 |
sphingosine and a fatty acid R group....ceramide
|
|
|
|
a ceramide
126 |
sphingosine and a fatty acid R group
|
|
|
|
sphingomyelin
|
phosphoraled ceramide
2/27 in lecture notes |
|
|
|
cerebroside
|
ceramide and a 6c sugar ring
|
|
|
|
ganglioside
|
ceramide and and 4 6c sugar rings
found in high concentrations in nervous system |
|
|
|
cholesterol
|
4 rings attached to each other...lipid
|
|
|
|
how rare or abundant is cholestoral?
|
extremely abundant in some eukaryotic membranes, such as the plasma membrane of mammalian liver, 1:1 molar ratio with other phospolipids
|
|
|
|
describe relationship between prokaryotic membranes and plant membranes relating to cholestoral
|
cholestoral is absent from both plant and prokaryotic membranes
|
|
|
|
two functions of cholestoral when in the membrane
|
-increase the permeability barrier and modulate membrane fluidity
|
|
|
|
integral membrane proteins
|
-hydrophobic section of the protein is imbedded in the hydrophoic core of the lipid bilayer-held by hydrophobic effects
-often extend all the way through the lipid bilayer..transmembrane proteins |
|
|
|
peripheral membrane proteins
|
-located outside lipid bilayer-held to polar surface of membrane by weak bonds
|
|
|
|
lipid anchored proteins
|
-located outside bilayer but are covalently bound to lipides that are part of the bilayer structure
|
|
|
|
3 types of membrane proteins
|
-integral membrane proteins
-peripheral membrane proteins -lipid anchored proteins |
|
|
|
crystals of membrane proteins which retain their native conformation are what?
|
difficult to obtain so only a relatively small umber of definitive tertiary structures are available via X-ray crystallography
because of this alternative methods were devised to look at structure of protein when embedded in membrane |
|
|
|
methods for studying integral membrane protein structure
|
1)freeze fracture electron microscopy
2)extracting integral proteins from membranes using nonionic detergents 3)limited enzyme digestion to determine the sidedness of the protein 4)hydropathicity plots 5)spatial relatiohships between amino acid residues in the transmembrane domain using cross-linking 6)electron paramagnetic spectroscopy |
|
|
|
studying integral membrane proteins using freeze fracture electron microscopy
|
Can resolve gross dimensions of membrane proteins, their density in the membrane and whether subunit (quaternary) structure exists. Cannot resolve details of tertiary structure.
|
|
|
|
using nonionic detergents to study integral membrane proteins
|
|
|
|
|
using limited enzyme digestion to determine the sidedness of the protein
|
|
|
|
|
identifying transmembrane domains through hydropathicity plots
134 draw plot |
identifies runs of hydrophobic amino acid residues as potential transmembrane domains
Hydrophobicity is measured by the free energy required to transfer each segment of the poly peptide from a nonpolar solvent to an aqueous medium peaks above the 0 are non-polar and have +delta G when placed in water. |
|
|
|
determining the spatial relationships between amino acid residues in the transmembrane domain using cross-linking
|
|
|
|
|
using electron paramagnetic spectroscopy to monitor changes in conformation
|
|
|
|
|
methods for studying peripheral membrane protein structure
method for studying anchored proteins |
|
|
|
|
what is Tc?
|
transition temperature
membrane has a uniuqe Tc at which the membrane freezes...transfromrs from liquid to a gel |
|
|
|
draw gel vs liquid structure and plot
|
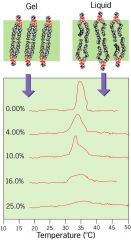
..
|
|
|
|
why does Tc vary membrane to membrane?
|
environment and functional needs
|
|
|
|
low Tc
|
more fluid bilayer at room temperature...further from freezing
higher percent of unsaturated fats short fatty acid chains |
|
|
|
what can cause low Tc in lipids?
|
-unsaturated fatty acid chains...double bonds or short fatty acid chains
- |
|
|
|
what does high cholesterol do to Tc
|
broadens temperature range over which gelling occurs....creates an intermediate fluidity in the temperature range
|
|
|
|
how to maintain or change lipid fluidity
|
Lipid fluidity can be controlled by synthesizing new phospholipids with different fatty acid properties
or remodeling existing phospholipidfatty acid chains using enzymes that insert/remove double bonds in the chain Fluidity can be maintained constant at different temperatures by this means –important in cold-blooded animals, like pond dwelling fish which experience extremes of temperature. Reduced temperatures in winter require a lower Tcto maintain membrane fluidity and so more unsaturated phospholipids are inserted into cell membranes |
remember lower Tc means more liquid just like a frozen pond will have the water portion lower than the ice(gel)
|
|
|
what about lipids with different headgroups or different lipids entirely
ex.. |
asymmetry between being found in cytosolic membrane and exoplasmic membrane.
ex.. sphingomyelin-extracellular phosphatidylcholine-extracell phospatidylserine-intracellul |
|
|
|
why is asymmetry maintained in lipids?
|
flip-flopping of lipids across the membrane is very energetically unfavourable...
very slow unless there is a flip-ase enzyme |
|
|
|
sumarize lipid mobility
|
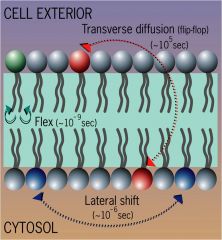
Lateral lipid diffusion – high – lipids can travel a micron in a 10^-6 seconds
Transverse diffusion – v. low |
|
|
|
what did fye and ediden discover?
|
demonstrated diffuesion of proteins within the plane of the membrane in chimeric cells created by fusion
|
ed paid a fee to get a mouse and a human
|
|
|
demonstrated diffuesion of proteins within the plane of the membrane in chimeric cells created by fusion
|
fye ediden
|
|
|
|
draw fye and ediden experiment
|
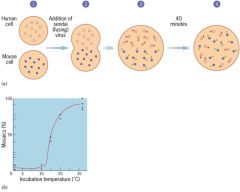
deminstrates diffusion of proteins within the plane of the membrane
1)common integral membrane protein in mouse cells labled with primary antibody and secondary fluorescent secondary antibody that emmits green light 2)same is done for a protein in human cells, but uses red light 3)fuse cells 4)after 40 minutes..fully mixed |
|
|
|
FRAP
|
fluorescence recovery after photobleaching
|
|
|
|
draw Frap experiment
|
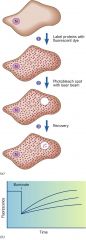
aa
|
fluorescent recovery after photobleaching
|

