![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
68 Cards in this Set
- Front
- Back
|
Why do chemicals form bonds |
They share or transfer outer electrons to get a more stable electron arrangement. |
|
|
Ionic bonding |
Includes metals and non-metals the metal has 1,2,3 electrons in outer shell so find it easier to just transfer electrons. They can form ionic lattices. |
|
|
Ionic lattice have a big melting point why |
Because there is a strong attraction between the oppositely charged ion, which means they require lots of energy to overcome. |
|
|
Structure of sodium chloride |
Ionic lattice. The sodium is positively charged and the chlorine is negatively charged so they have a strong attraction to each other, and are held together by the electrostatic forces. |
|
|
Properties of ionic compounds |
Are solid at room temperature. Have giant structures. High melting points. They conduct electricity when dissolved or molten because the charged ions can move. They are brittle and shatter contact with like charges. |
|
|
Covalent bonding |
Two non-metals share a pair of electrons to get a full outer shell. |
|
|
How does sharing electrons hold atoms together |
Held by electrostatic forces of attraction. |
|
|
Properties of substances with molecular structures |
Some have strong covalent bonds so are solid at room temperature. The gases and liquids have weaker van der waals forces of attraction. Poor conductors of electricity as don't have charge. Some dissolve in water others don't |
|
|
Co-ordinate bonding |
One atom donates both pair of electrons from its own lone pair. They have a charge because of donated pair. |
|
|
Electronegativity |
Is the ability of an atom to draw the electron density in a covalent bond to itself. |
|
|
Scale used to erasure the electronegativity |
Pauling scale goes from 1to 4 |
|
|
Most electron negative |
Fluorine |
|
|
Electronegativity depends on |
The nuclear charge distance form the nucleus and the outer shell the shielding
note smaller atom more nuclear charge more electronegative the larger the nuclear charge the grater the electronegativity
|
|
|
Trends in elctronegativity |
Down a group less elctronegative because more shielding across a period more electronegative more nuclear charge no increase in shielding. |
|
|
Polarity of covalent bonds |
Polar means unequal sharing of the bonds it is a bond property. |
|
|
Covalent bonds between two atoms that are the same |
The electron density is equally distributed so it's non-polar . |
|
|
Covalent bonds between two atoms that are different |
They are polar one is more electronegative. Remember delta , positive and negative |
|
|
Metallic bonding |
They are attracted to same metal to make an metal ion. They make a sea of delocalised electrons which has strong nuclear attraction to the positive nucleus. |
|
|
Properties of metals |
Can carry a charge because of delocalised electrons have high melting points because attraction between the nucleus and the electrons that are negatively charged. They are strong because of their charge bigger charge the more attraction: the size of the ion :smaller ion closer to nucleus higher attraction. Metal are malleable and ductile as they are in the same environment when pulled or street he'd as electrons can move. |
|
|
Forces between molecules |
Van der waals are the weakest permenant dipole dipole hydrogen bonding the strongest. The strongest
|
|
|
Van der waals forces |
The weakest forces in every atom more that there are the higher the melting point more electrons more van der waals forces . |
|
|
Dipole dipole |
These are fond in those that have a big differences in electronegativity. They flip so that they are the opposite charge one would be delta +other delta-. |
|
|
Hydrogen bonding |
Hydrogen is bonded to very electronegative element atom to make strong partial charge. These have lone pair of electrons that are attracted to the positive partial charge of the hydrogen. |
|
|
The boiling points of hydrides |
FONding the ends have the highest boiling point it then decreases a lot but increases down the group has there are more van der waals forces of attraction. |
|
|
Importance of hydrogen bonds |
They can be made or broken under conditions that don't effect covalent bonds. |
|
|
The structure and density of ice |
The molecules can't move as they are solid. So make cube like structures the hydrogen bonds stay the same but become more spread out and making air sacks that make the ice act as an insulator locking heat into the water in winter. |
|
|
Crystals |
These have a regular arrangement and are held together by forces of attraction. These could be intermolecular or bonds. |
|
|
The types of crystals |
Ionic Macromolecular Simple molecular Metallic |
|
|
Ionic crystals or lattice |
These have strong ionic attractions in between the oppositely charged ions and meaning they have high melting points, s the attraction is hard to overcome. |
|
|
Metallic crystal or lattice |
Positive ions in negatively charged sea of delocalised electrons. High melting points result from the strong attraction of the positive ion and negative electron. |
|
|
Graphite |
This is made of pure carbon but the atoms are bonded and arranged differently than in a diamond. Graphite has van der waals forces and strong covalent bonds. Each carbon makes three covalent bonds due to repulsion these make a flat trigonal planar,leaving one free to carry a charge. This makes layers that can slide over each other. The layers are held together by weak van der waals forces of attraction. Graphite is soft it has high melting point and can conduct electricity along the planes of hexagons. |
|
|
The structure and density of ice |
The molecules can't move as they are solid. So make cube like structures the hydrogen bonds stay the same but become more spread out and making air sacks that make the ice act as an insulator locking heat into the water in winter. |
|
|
Crystals |
These have a regular arrangement and are held together by forces of attraction. These could be intermolecular or bonds. |
|
|
The types of crystals |
Ionic Macromolecular Simple molecular Metallic |
|
|
Ionic crystals or lattice |
These have strong ionic attractions in between the oppositely charged ions and meaning they have high melting points, s the attraction is hard to overcome. |
|
|
Metallic crystal or lattice |
Positive ions in negatively charged sea of delocalised electrons. High melting points result from the strong attraction of the positive ion and negative electron. |
|
|
Molecular crystals |
Two non-metals held together by intermolecular forces, covalent bonds act between the atoms but not the molecules, so they have low melting points due to the weak forces of attraction. |
|
|
Properties of molecular structures |
Soft and break easily Low melting temperature Don't carry heat or current |
|
|
Macromolecular crystals |
These are giant covalent compounds with high melting points. |
|
|
Diamond |
It's a macro molecular structure with strong covalent bonds between every carbon atom. All four of its electrons make covalent bonds with other carbons making a giant structure. It is very hard, has a high melting point but doesn't conduct electricity because there are no free electrons. |
|
|
definition of ionic bonding
|
an ionic bond is the electrostatic force of attraction between oppositely charged ions formed by electron transfer
|
|
|
patterns in metal and non-metals in gaining and losing electrons |
metal atoms lose electrons to form positive ions and nonmetals atoms gain electrons to become negative ions |
|
|
when is ionic bonding stronger |
ionic bonding is stronger when ions are smaller and have higher charges. |
|
|
definition of a covalent bond |
a covalent bond is a shared pair of electrons |
|
|
what is a codative bond where do the electrons come from draw common coordinate bond |
A Dative covalent bond forms when the shared pair of electrons in the covalent bond come from only one of the bonding atoms. A dative covalent bond is also called co-ordinate bonding. Common examples you should be able to draw that contain dative covalent bond (e.g. NH4 + , H3O+ , NH3BF3 ) The dative covalent bond acts likean ordinary covalent bond whenthinking about shape so in NH4+the shape is tetrahedral
|
|
|
define metallic bonding |
A metallic bond is the electrostatic force of attraction between thepositive metal ions and the delocalised electrons
|
|
|
the main factors that effect the strength of a bond |
The three main factors that affect the strength of a metallic bond are: 1. Number of protons/ Strength of nuclear attraction. The more protons the stronger the bond 2. Number of delocalised electrons per atom (the outer shell electrons are delocalised) The more delocalised electrons the stronger the bond 3. Size of ion. The smaller the ion, the stronger the bond.
|
|
|
give an example of effect of factors using Mg and Na |
Mg has stronger metallic bonding than Na and hence a higher melting point. The Metallic bonding gets stronger because in Mg there are more electrons in the outer shell that are released to the sea of electrons. The Mg ion is also smaller and has one more proton. There is therefore a stronger electrostatic attraction between the positive metal ions and the delocalised electrons and higher energy is needed to break bonds. |
|
|
Bonding and StructureGiant Ionic LatticeBonding Structure Examples
|
Ionic : electrostatic force of attraction between oppositely charged ions Sodium chloride Magnesium oxide
|
|
|
Bonding and Structure covalent simple molcular Bonding Structure Examples
|
Covalent : shared pair of electrons Simple molecular: With intermolecular forces (van der Waals, permanent dipoles, hydrogen bonds) between molecules Iodine Ice Carbon dioxide Water Methane
|
|
|
Bonding and Structure covalent and marcomolecular Bonding Structure Examples
|
Covalent : shared pair of electrons Macromolecular: giant molecular structures. Diamond Graphite Silicon dioxide Silicon
|
|
|
Bonding and Structure metallic Bonding Structure Examples
|
Metallic: electrostaticforce of attraction betweenthe metal positive ions andthe delocalised electrons Giant metalliclatticeMagnesium, Sodium(all metals)
|
|
|
Ionic
Property boiling andmeltingpoints Solubility inwater conductivitywhen solid conductivitywhen molten generaldescription |
high- because of giant lattice of ions with strong electrostatic forces between oppositely charged ions.
Generallygood poor: ionscan’t move/fixed in lattice good: ions canmove crystallinesolids |
|
|
Molecular (simple) boiling and melting points Solubility in water conductivity when solid conductivity when molten general description |
low- because of weak intermolecular forces between molecules (specify type e.g van der waals/hydrogen bond)
generally poor poor: no ions to conduct and electrons are localised (fixed in place) poor: no ions mostly gases and liquids |
|
|
Macromolecular boiling and melting points Solubility in water conductivity when solid conductivity when molten general description |
high- because of many strong covalent bonds in macromolecular structure. Take a lot of energy to break the many strong bonds
insoluble diamond and sand:poor, becauseelectrons can’t move(localised)graphite: good as freedelocalised electronsbetween layers poor solids |
|
|
Metallic boiling and melting points Solubility in water conductivity when solid conductivity when molten general description |
high- strong electrostatic forces between positive ions and sea of delocalised electrons
insoluble good: delocalised electrons can move through structure good shiny metal Malleable as the positive ions in the lattice are all identical. So the planes of ions can slide easily over one another -attractive forces in the lattice are the same whichever ions are adjacent |
|
|
how to explain shape in a question |
1. State number of bonding pairs and lone pairs of electrons.2. State that electron pairs repel and try to get as far apart as possible (or to aposition of minimum repulsion.)3. If there are no lone pairs state that the electron pairs repel equally4. If there are lone pairs of electrons, then state that lone pairs repel more thanbonding pairs.5. State actual shape and bond angle.
|
|
|
bonding and lone pairs |
Remember lone pairs repel more than bonding pairs and so reduce bond angles (by about 2.5oper lone pair in above examples) Occasionally more complex shapes are seen that are variations of octahedral and trigonal bipyramidal where some of the bonds are replaced with lone pairs. You do not need to learn the names of these but ought to be able to work out these shapes using the method below e.g XeF4 e.g. BrF5 e.g I3 - e .g.ClF3 e.g. SF4 & IF4 + :X X X: : : X : : Xe has 8 electrons in its outer shell. 4 F’s add 4 more electrons. This makes a total of 12 electrons made up of 4 bond pairs and 2 lone pairs. The means it is a variation of the 6 bond pair shape (octahedral) Cl has 7 electrons in its outer shell. 3 F’s add 3 more electrons. This makes a total of 10 electrons made up of 3 bond pairs and 2 lone pairs. The means it is a variation of the 5 bond pair shape (trigonal bipyramidal) I has 7 electrons in its outer shell. 4 F’s add 4 more electrons. Remove one electron as positively charged. This makes a total of 10 electrons made up of 4 bond pairs and 1 lone pair. The means it is a variation of the 5 bond pair shape (trigonal bipyramidal) Square planar Bond angle 90O Bond angle ~89O (Reduced by lone pair) Bond angle 180O Bond angle ~89O (Reduced by lone pairs) Bond angles ~119 + 89O (Reduced by lone pair
|
|
|
define electronantivity and elements that take part in hydrogen bonding |
Electronegativity is the relative tendency of an atom in a covalent bondin a molecule to attract electrons in a covalent bond to itself.F, O, N and Cl are themost electronegativeatoms.
|
|
|
factors effecting electronegaitivity |
Factors affecting electronegativityElectronegativity increases across a period as the number of protons increases and the atomic radiusdecreases because the electrons in the same shell are pulled in more.It decreases down a group because the distance between the nucleus and the outer electrons increases andthe shielding of inner shell electrons increasesA compound containing elements of similar electronegativity and hence a smallelectronegativity difference will be purely covalent
|
|
|
Formation of a permanent dipole – (polar covalent) bond
|
A polar covalent bond forms when the elements in the bond have different electronegativities. When a bond is a polar covalent bond it has an unequal distribution of electrons in the bond and produces a charge separation, (dipole) δ+ δ- ends. The element with the larger electronegativity in a polar compound will be the δ- end H – Cl δ + δ – A compound containing elements of very different electronegativity and hence a very large electronegativity difference will be ionic. If all the bonds in a compound are the same polar bond and there are no lone pairs then the dipoles cancel out and the substance will be non polar. e.g. CCl4 will be non-polar whereas CH3Cl will be polar
|
|
|
Intermolecular bondingVan der Waals’ Forces
|
Van der Waals forces occur between all molecular substances and noble gases.They do not occur in ionic substances.
These are also called transient, induced dipole-dipole interactions. They occur between all simple covalent molecules and the separate atoms in noble gases. In any molecule the electrons are moving constantly and randomly. As this happens the electron density can fluctuate and parts of the molecule become more or less negative i.e. small temporary or transient dipoles form. These instantaneous dipoles can cause dipoles to form in neighbouring molecules. These are called induced dipoles. The induced dipole is always the opposite sign to the original one. |
|
|
Main factor affecting size of Van der waals
|
The more electrons there are in the molecule the higher the chance that temporary dipoles will form. Thismakes the van der Waals stronger between the molecules and so boiling points will be greater.
|
|
|
the effect of size and VDW |
The increasing boiling points of the alkane homologous series can be explained by the increasing number of electrons in the bigger molecules causing an increase in the size of the van der Waals between molecules. Permanent dipole bonding occurs in addition to van der waals forces The increasing boiling points of the halogens down the group 7 series can be explained by the increasing number of electrons in the bigger molecules causing an increase in the size of the van der Waals between the molecules. This is why I2 is a solid whereas Cl2 is a gas. The shape of the molecule can also have an effect on the size of the van der Waals forces. Long chain alkanes have a larger surface area of contact between molecules for van der waals to form than compared to spherical shaped branched alkanes and so have stronger VDW
|
|
|
Permanent dipole-dipole bonding
|
•Permanent dipole-dipole bonding occurs between polar molecules •It is stronger than van der waals and so the compounds have higher boiling points •Polar molecules have a permanent dipole. (commonly compounds with C-Cl, C-F, C-Br H-Cl, C=O bonds) •Polar molecules are asymmetrical and have a bond where there is a significant difference in electronegativity between the atoms. Permanent dipole bonding occurs in addition to van der waals forces
|
|
|
hydrogen bonding where does it occur and what elements are involved things to remember when drawing
the strength of the bond |
It occurs in compounds that have a hydrogen atom attached to one of the three most electronegative atoms of nitrogen, oxygen and fluorine, which must have an available lone pair of electrons. e.g. a –O-H -N-H F- H bond. There is a large electronegativity difference between the H and the O,N,F
Hydrogen bonding occurs in addition to van der waals forces Always show the lone pair of electrons on the O,F,N and the dipoles and all the δ - δ + charges Hydrogen bonding is stronger than the other twotypes of intermolecular bonding. |
|
|
hydrogen bonding pattern and the reasons |
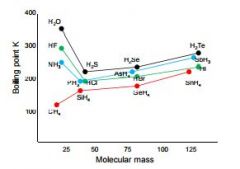
The anomalously high boiling points of H2O,NH3 and HF are caused by the hydrogenbonding between the moleculesThe general increase in boiling point from H2Sto H2Te is caused by increasing van der Waalsforces between molecules due to an increasingnumber of electrons.Alcohols, carboxylic acids, proteins, amides allcan form hydrogen bonds
|
|
|
four types of crystal structure |
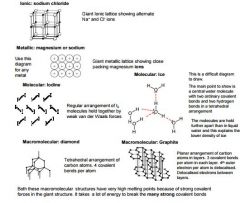
|

