![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
|
Which two metabolic pathways are upregulated in response to decreased nutrient intake? |
Gluconeogenesis Ketogenesis/ketolysis |
|
|
What is gluconeogenesis? |
Synthesis of glucose from non-carbohydrate precursors |
|
|
Give some common substrates for gluconeogenesis? |
Lactate Glycerol Amino acids such as alanine and glutamine |
|
|
Where does gluconeogenesis occur? |
In the liver, and also slightly in the kidney |
|
|
Which cellular compartments are used in gluconeogenesis? |
Mitochondria and cytosolic + a small ER section |
|
|
Which tissues cannot utilise fat as a fuel source? |
Brain RBCs Renal medulla |
|
|
What is the daily glucose requirement of the brain? |
110g |
|
|
What is the daily glucose requirement of muscle? |
30g |
|
|
What is the daily glucose requirement of the renal medulla? |
30g |
|
|
What is the daily glucose intake of red blood cells? |
25g |
|
|
What is the recommended daily glucose intake? |
~100g |
|
|
How many grams glucose are present in the blood? |
15g |
|
|
How long do glycogen stores last during a fasted state? |
12-16 hours |
|
|
For how long after a meal is exogenous glucose the main source of glucose? |
4 hours |
|
|
Glycogen is the bodies main source of glucose during which time period after a meal? |
4-16 hours |
|
|
Gluconeogenesis is the bodies main form of glucose during which time period after a meal? |
16 hours - 2 days |
|
|
Which other pathway is gluconeogenesis similar to? Give examples of some differences |
Glycolysis - the irreversible reactions in glycolysis require different enzymes in order to reverse them - Gluconeogenesis requires 6 ATPs, while glycolysis produces 2 - they have different control mechanisms - Gluconeogenesis involves the mitochondria as well as the cytoplasm |
|
|
What is the goal of the mitochondrial stage of gluconeogenesis? Why does this stage have to happen at all? |
To convert pyruvate into phosphoenolpyruvate. Because the reverse is an irreversible reaction in glycolysis, another pathway is required to get around the 'block' |
|
|
Write out the sequence of reactions required to convert pyruvate to phosphoenolpyruvate |
- imported into mitochondria - pyruvate + ATP => OAA (pyruvate carboxylase) - OAA + NADH => Malate + NAD+ + H+ (malate dehydrogenase) - malate exported into cytosol - malate + NAD+ + H+ => OAA + NADH (malate dehydrogenase) - OAA + GTP => PEP (PEP carboxykinase) |
|
|
Which 3 enzymes found in glycolysis are not found in gluconeogenesis and why? |
Pyruvate kinase Phosphofructokinase-1 Glucokinase These are all the irreversible reactions of glycolysis, so to go backwards you need a new enzyme |
|
|
Which enzyme takes the place of Phosphofructokinase-1 in gluconeogenesis? Write out the equation that uses this enzyme |
Fructose-1,6-bisphosphatase F16BP => F6P |
|
|
Which enzyme replaces Glucokinase in gluconeogenesis? Write out the equation that involves this enzyme. Where does this reaction occur? |
Glucose-6-phosphatase G6P => glucose Takes place in ER |
|
|
How many energy molecules are required to produce one molecule of glucose from gluconeogenesis? |
4 ATPs 2 GTPs |
|
|
How is lactate incorporated into the gluconeogenic pathway? |
Lactate + NAD+ => Pyruvate + NADH catalysed by lactate dehydrogenase |
|
|
How is alanine incorporated into the gluconeogenic pathway? |
Alanine => Pyruvate + NH3 Catalysed by alanine aminotransferase |
|
|
Where is lactate produced? |
Anaerobically respiring muscle RBCs Renal medulla |
|
|
How is glutamine incorporated into the gluconeogenic pathway? |
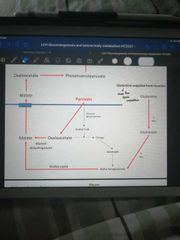
Glutamine => glutamate +NH3 Glutamate => @ketoglutarate + NH3 @ketoglutarate moves around the Krebs cycle to malate, where it is exported as part of gluconeogenic pathway |
|
|
How is glycerol incorporated into the gluconeogenic pathway? |
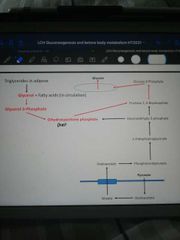
Glycerol => glycerol-3-phosphate G3P => DHAP, which is in gluconeogenic pathway (in equilibrium with glyceraldehyde-3-phosphate |
|
|
Why can you make glucose out of triglycerides but not fatty acids? |
Fatty acids contribute AcCoA to the Krebs cycle, adding 2 carbons. However, those two carbons are lost as CO2 as the Krebs cycle turns, negating their effect. Triglycerides can be utilised for their glycerol, but the fatty acids are useless |
|
|
How is fructose-1,6-bisphosphatase acutely regulated? |
Stimulated by citrate Indirectly stimulated by glucagon |
|
|
How is PEP carboxykinase acutely regulated? |
Stimulated by glucagon (GPCR activation) |
|
|
How is pyruvate kinase acutely regulated? |
Inhibited by glucagon (GPCR activation) |
|
|
How is pyruvate carboxylase acutely regulated? |
Stimulated by AcCoA |
|
|
How do hormones regulate the enzymes in gluconeogenesis and glycolysis? |
They affect transcription of the enzymes and so increase or decrease their production |
|
|
Which gluconeogenic/glycolytic enzymes are upregulated by glucagon/glucocorticoids? |
Pyruvate carboxylase PEP carboxykinase Fructose-1,6-bisphosphatase Glucose-6-phosphatase |
|
|
Which gluconeogenic/glycolytic enzymes are downregulated by glucagon/glucocorticoids? |
Glucokinase Pyruvate kinase |
|
|
Which gluconeogenic/glycolytic enzymes are upregulated by insulin? |
Glycogen synthase Hexokinase Phosphofructokinase Pyruvate kinase |
|
|
Which gluconeogenic/glycolytic enzymes are downregulated by insulin? |
Pyruvate carboxylase PEP carboxykinase Fructose-1,6-bisphosphatase Glucose-6-phosphatase |
|
|
How long into fasting does gluconeogenesis start to decrease? |
Around 2 days |
|
|
What are Ketones? |
Small, 4 carbon molecules Water-soluble |
|
|
What are the two main metabolically active Ketones? |
Acetoacetate beta-hydroxybutyrate |
|
|
Which two Ketones are produced from acetoacetate, and what are the types of reaction that produce them? |
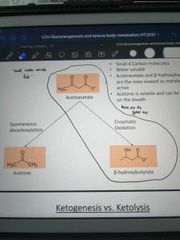
Acetone by spontaneous decarboxylation Beta-hydroxybutyrate by enzymatic oxidation |
|
|
What is Ketogenesis and where does it occur? |
Production of Ketone bodies Occurs in the mitochondria of liver cells |
|
|
What is ketolysis? Where does it occur? |
Breakdown and utilisation of Ketone bodies Occurs in the mitochondria of peripheral tissues such as CNS during starvation |
|
|
Why does ketolysis not occur in the liver? |
Because it would be wasteful for the liver to produce Ketone bodies just to break them down again, futile cycle |
|
|
What are the precursors of the starter molecule for ketogenesis? Where do they come from and how do they get to the liver? |
NEFAs Released from adipocytes due to lack of insulin which activates hormone sensitive lipase |
|
|
What is HSL and how does it work? |
Hormone-sensitive lipase, which regulates lipolysis. Can be activated by phosphorylation |
|
|
Once the NEFAs arrive at the liver, what happens to them? |
They are broken down in the exact same way as in beta-oxidation, into AcCoA |
|
|
Write out the reactions required for ketogenesis, starting with two AcCoAs. |
- 2 AcCoA => Acetoacetyl coA (Acetoacetyl coA thiolase) - Acetoacetyl coA + AcCoA => HMG-coA (HMG-coA synthase) - HMG-coA => Acetoacetate + AcCoA (HMG-coA lyase) Acetoacetate is the principal ketone |
|
|
Write the reaction that converts Acetoacetate into beta-hydroxybutyrate |
Acetoacetate + NADH => Beta-HB + NAD+ Beta-HB dehydrogenase |
|
|
What drives the diversion of AcCoA into ketogenesis? |
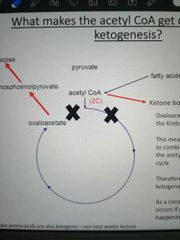
Because OAA is being diverted into the gluconeogenic pathway, the amount that can combine with AcCoA to restart the Krebs cycle goes down. This leads to a buildup of AcCoA that can't go through the Krebs cycle, so it undergoes ketogenesis instead |
|
|
What must also be occurring for ketogenesis to take place? |
Gluconeogenesis |
|
|
Which enzyme is lacking in the liver that means it can't carry out ketolysis? |
3-ketoacyl CoA Transferase |
|
|
Write out the reactions of ketolysis, starting from beta-HB |
- Beta-HB + NAD+ + H+ => Acetoacetate + NADH (B-HB dehydrogenase) - acetoacetate + succinyl coA => Acetoacetyl coA + succinate (3-ketoacyl CoA transferase) - Acetoacetyl CoA + CoASH => 2x AcCoA (Acetoacetyl CoA thiolase) |
|
|
What is diabetic ketoacidosis? |
When, as a result of type 1 diabetes, your tissues can't take up glucose, resulting in high Ketone body concentrations to provide an emergency fuel source Ketones are acidic, so they lower the blood pH, which can lead to coma and death |

