![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
122 Cards in this Set
- Front
- Back
|
development of proper growth depends on many things - name 6
give an example of a drug that can cause increased RV afterload and tricusp regurg in the fetus (possible stillbirth) in contrast to a premature/Trisomy/rubella infant... |
insulin, Epidermal growth factor, and other hormones
and substrate ex: if you don't have enough insulin --> intrauterine growth restriction if you have too much insulin --> Macrosomia OTHER: -genetic (Aneuploidy, race, gender) -fetal factors (regulatory and counter regulatory hormone balance, receptor maturation) -maternal environment (uterine, placental flow[placenta is NOT a passive organ - it as a lot of protective active fct], anatomy and fct) -macroenvironment [infectious diseases (TORCHES), exposures (tobbacco, alcohol, radiation, teratogens))] fetal heart problem: ex: took some advil --> closed the patent ductus, so no more R--L shunt! [normally, the ductus shuts reversibly immediately with the first breath because the pressure in the left atrium drops below that of the right - then with the incerase in oxygen in the baby, the prostaglandin levels drop and the duct closes permanently by month 1 - aspirin will decrease the prostaglandin] Premature, Trisomy18/13, congenital rubella: can cause patent ductus arteriosus. This will cause the increased work of breathing and poor weight gain. With age, the PDA may lead to congestive heart failure. If this goes on, the heart compensates by getting stronger, we can see an Eisenmenger syndrome with a reversal of the shunt direction (from a L-->R shunt to a R-->L shunt) --> cyanosis |
|
|
5 major substrates needed for a fetus?
(compare to the 4 major substrates adequate for growth of infant) what are the major hormones? |
Oxygen
Glucose Lactate Amino acids Micronutrients Infant: Lipids, Carbohydrates, Proteins, Micronutrients hormones: -insulin -Epidermal growth factor -Insulin-like Growth factor I and II |
|
|
why is breastmilk best
|
nutritionally: suitable caloric density, high quality adequate mineral content, low renal solute load [hypoCA of infancy - with formula fed babies the PO4 is too high and Ca goes down -- seizure ], proteins are higher quality - easier to digest
Immunologically: antibodies and macrophages for protection against GI pathogens and possibly deleterious organisms Psychologically: bonding effect occurs during breast feeding. with breast milk: there is less incidence of diarrhea URI, OM and reduced incidence of allergic diseases, lymphoma, Crohns, DMtypeI, neurologic disability |
|
|
only nutrient "not supplied enough" in breast milk
it's complete nutrient source for 4-5months, possibly except for this |
vitamin D
Fe content is also low, but very bioavailable - so there is no need to supplement Fe (if you give them Fe, it helps bacteria to grow - so it's actually worse to give them some) |
|
|
when does breast milk come?
|
4-5days or so
|
|
|
possible problems during breastfeeding
|
breastfeeding jaundice - essentially from dehydration (the milk is very concentrated and high in protein/lipids.. then the blood becomes more concentrated and he get's jaundice)
breastmilk jaundice -there is something in the milk that sustains the jaundice - that's considered physiologic - this breast milk factor prevent the jaundice metabolism contraindications to breastfeeding -infectious diseases (active TB, HIV, HTLV) BUT, staph aureus- it's not a contraindication to stop feeding - it's probably better to keep feeding -Drugs (anti-cancer drugs, anti-retrovirals, illicit) |
|
|
If formula fed,
changing from cow to soy? cow milk problem? balanced diet? |
cow milk-based formulas and soy-based formulas are commercially available
you can get a cow protein allergy (bloody diarrhea, poor weight gain) -no cow milk before the end of the 1st month but the chance of getting an allergy to the soy protein is about the same !! so don't just switch them to soy diet: 20-30% from fat 10-30% from protein 40-60% from carbs (complex!) |
|
|
developmental aspects of body composition
when does the fetus double it's weight? how does he do it? how much of the first 1.5kg of weight is due to fat compared to the second? what is laid down in the last few weeks? |
in the last trimester the fetus doubles its weight due to deposition of protein and fat
of the first 1.5kg of body weight, only 5g are fat, while 2nd 1.5kg, 500g is fat (regulated by insulin + nutrient supply) during the last few weeks of the 3rd trimester, stores of Cu, Fe, Zn and Vitamin A are laid down |
|
|
various compartments
compare 16wk fetus to term baby to 3month old |
16 week: 2/3 of weight is Extracellular water, 20% is intracell, and 13% is non-water
Term: 40% Extracellular water, 40% is intracell, and 20% is non-water as the baby grows, the non--water compartment increases faster so at 3 months: 15% ECW 45% ICW 40% Non-Water |
|
|
Regulation of fetal growth
it's an increase in size and complexitiy involving changes in .... growth velocity is highest for length at... and for weight at... |
Growth involves changes in both cell number and size:
Growth velocity (fractional change per unit time) is greatest for length at 20 weeks and for weight at 34 weeks. Variation in birth weight is due approximately 1/3 to genetic factors, 1/3 to environmental factors, and 1/3 unknown. |
|
|
fetal caloric requirements
besides the baby and the mom, what is suppled? |
95kcal/kg/day of which 55kcal/kg/day are oxidized, and 40kcal/kg/day are required for growth
taking into account the mother's anabolism as well, the extra caloric intake needed is 100-200kcal/day The placenta grows faster than the fetus: Reaches maximum weight at 33 weeks. The placenta consumes up to 50% of the glucose and O2 delivered to it via the maternal circulation. |
|
|
placenta takes what??
|
It is NOT a passive organ.
50% of glucose and oxygen delivered to it via the maternal circulation placenta grows faster than fetus reaching mac weight at about... |
|
|
postnatal growth
what calories are needed what if it's too much/too little |
Nutrition is the key environmental factor affecting growth:
Normal infant needs at least 120 kcal/kg/day to achieve optimal growth Malnourished children show: Decreased growth rate Delayed bone age. Delayed puberty. Short adult height. Undernutrition retards brain development and immune function. Overnutrition results in: Obesity. Increased growth rate. Slightly earlier puberty. Slightly decreased adult height. |
|
|
If nutrient demand exceeds supply, body redirects
|
If nutrient demand exceeds supply, the body re-directs growth to assure survival:
Brain growth and cardiovascular function take priority over visceral and somatic growth The brain constitutes about 10% of body weight at birth, and completes its growth by age 2 years. |
|
|
regulation of post-natal growth - what hormone is needed when?
|
growth during infancy depends on nutrition, growth hormone, and thyroid H
in childhood: GH governs growth growth in pubertal phase: Sex hormones Linear growth: Depends on hormonally-regulated chondrocyte proliferation in the growth plate. Hormones that affect chondrocyte proliferation include: Growth Hormone Thyroid Hormone Insulin Glucocorticoids And Sex Steroids |
|
|
IUGR
symmetric? |
intrauterine growth restriction
-this means PATHOLOGY - it implies that something happened (either not eough supply, processing or delivery) -normal developing fetus and then it get's cut off from nutrition, the first thing is going to be loss of weight gain -if this persists, the second thing is length -then it's head growth symmetric is either: congenital infx or genetic potential for growth Slide: Implies pathology: must be differentiated from SGA Major cause: inadequate delivery of substrates to the fetus (abnormalities in utero-placental unit) Other causes: Infectious agents (STORCH) Genetic (aneuploidy) Toxins (alcohol, drugs, tobacco) Maternal diseases (pre-eclampsia, hypertension, SLE, hypercoagulable states) Either symmetric (head, length and weight are equally affected) or asymmetric (weight is most affected, length less affected, and head circumference least affected) Symmetric implies either: congenital infection or low genetic potential for growth Asymmetric implies: chronic malnutrition and implicates the placenta Outcomes are generally good, provided there is no genetic or infectious cause |
|
|
Diabetic mom infant
why hypoglycemic later? all large except? long term effects? |
5% of all admissions to NICU
poor control of DM in 2nd and 3rd trimesters of pregnancy results in accelerated fetal growth and macrosomia (BW >4kg) Pedersen Hypothesis Maternal hyperglycemia --> Fetal hyperglycemia --> Premature maturation of fetal pancreatic islets with hypertrophy of b-cells --> Fetal hyperinsulinemia Fetal hyperinsulinemia results in excessive growth, especially of insulin-sensitive tissues: liver, skeletal and cardiac muscle, and subcutaneous fat Increased metabolic activity in this abnormally fast growing tissue leads to increased oxygen consumption --> relative fetal hypoxemia triggers erythropoietin --> fetal polycythemia the xs tissue is metabolically active --> infant has increase oxygen demand --> EPO --> fetal polycythemia --> risk for spontanous thrombus formation, strokes intrauterine or postnatally Two reasons for neonatal hypoglycemia: 1) Fetal hyperinsulinemia (as above), now without a steady glucose source 2) Depressed counter-regulatory hormonal response (glucagons, catecholamines) to hypoglycemia in IDM IDM can be macrosomic among all classes of IDM, except in the most severe class; here, diabetes causes a placental vasculopathy, causing IUGR Long term effects on the offspring of diabetic mothers: Hyperglycemia and hyperinsulinemia --> accelerated growth and abnormal glucose tolerance in later life Up to 50% of IDM who were LGA at delivery were heavier (but not taller) at 5-8 years of age compared to normal peers -This association extends to obesity in adolescence |
|
|
when is DM mom baby small?
|
Infants of DM moms, will get hyposomic macrosomic infants in all classes of IDM, except in moe severe class: here, DM causes placental vasculopathy, causing IUGR
|
|
|
drug use in children
intro |
off-label drug use is common, but there is no safety data available
NICU pt get 8.4-13dosease/day ADR rate 6% in unlicensed/off-label use ex: sildenafil (relaxes pulmonary bed) |
|
|
absorption
|
age/gastric pH effect important (phenobarital for neonatal seizures; infant can't go to pH 4.5, so the phenobarb is not absorbed as well)
food affect can be critical (FeSO4) absorption can be affected by other medications (H2 blockers, PPI) ex: meclopramide (motility agent -reglan) is not very beneficial because the receptors are not developed. ex. zantac - we decrease the H+ barrier and reducing the immune function - so we don't usually treat the happy spitters unless to appease parents |
|
|
drug distribution
|
Volume of distribution is a concept used to explain the drug concentrations we achieve (C 1/Vd)
Magnitude of Vd provides insight into drug distribution Large Vd → absorption in fat or protein binding Small Vd → distribution in plasma only Distribution can be affected by pathology (ascites, effusions, etc) [ex hydrops has massive edema] |
|
|
Metabolism in children
|
Liver is the drug organ
Liver can activate or inactivate Metabolites may be more important (therapeutic or toxic) than drug Important to be aware of interactions Two major types of drug metabolism Phase I: non-synthetic reactions Oxidation, reduction, hydrolysis Phase II: synthetic reactions Glucuronidation, sulfation, acetylation |
|
|
Cytochrome P450 (CYP) Basics
|
Superfamily of Phase 1 enzymes (oxidation, demethylation)
Nomenclature: CYP3A4 (3 = family, A = subfamily, 4 =Isoform) 17 Families and 39 subfamilies in humans CYP1, CYP2, CYP3 are primary drug metabolizing enzymes Half of all drugs metabolized by CYP3A subfamily CYP21 2C - 21 hydroxylase in the steroid pathway remember at least CYP3A |
|
|
(ml/min/kg or L/hr/m2)
|
elimination
|
|
|
Clearance and Drug Exposure
|
concentration as a function of time curve:
area under the curve = AUC = AUC is used to quantify exposure to a drug Clearance determines exposure AUC (Exposure) = Dose/CL AUC can be calculated just like the name implies (AREA UNDER THE CURVE …) AUC is used to determine bioavailability Bioavailability = AUCoral/ AUCi.v. |
|
|
Pharmacodynamics
what's the endpoint? |
Different than pharmacokinetics (=how drug changes) the dynamics is how the body is changed by the drug
Dynamics Drug Does Endpoints – NOT drug levels; the endpoints are what the patient is actually doing Examples How much change in BP do we see? How much does seizure frequency change? How much does ANC change? |
|
|
can we take an adult dose and change it to pediatric by weight?
|
NO, they have a different body composition and chemistry
ex - not as much fat... Children are NOT little adults Infants are NOT miniature children Very Low Birth Weight (VLBW; <1500g) neonates are NOT miniature term babies Scaling adult doses based on body weight or surface area does not account for developmental changes that affect drug disposition or tissue/organ sensitivity. Pharmacologic impact of developmental changes are often discovered when unexpected or severe toxicity in infants and children leads to detailed pharmacologic studies. Liver & kidney development has the greatest impact on drug disposition (pharmacokinetics) The most dramatic changes occur during the first days to months of life Effect of development on tissue/organ sensitivity to drugs (pharmacodynamics) is poorly studied |
|
|
Renal Ontogeny
how is the GFR at birth? changes? how is the tubular function? |
Glomerular filtration rate changes rapidly and then plateaus:
Low at birth -Full term newborn - 10-15 ml/min/1.73m2 -Premature - 5-10 ml/min/1.73m2 GFR doubles by 1 week of age Adult values by 6-12 months of age Tubular function changes linearly: Secretory function impaired at birth Adult values by 1 year of age normal adult GFR = 100ml/min/1.73m2 the newborns GFR change and fluctuate, you have to monitor the blood - can't just get one check; |
|
|
Hepatic Development - how are the phases at birth compared to adult?
compare the capacity to oxidize, glucuronate, sufonate, and acetylate at birth compared to adults |
Phase 1
-Activity low at birth -Mature at variable rates **Oxidative metabolism increases rapidly after birth **Alcohol dehydrogenase reaches adult levels at 5 yrs Phase 2 (conjugation, acetylation, methylation) Conjugation: Glucuronidation ¯ at birth Sulfatation at birth Acetylation ¯ at birth, “fast” or “slow” phenotype by 12-15 mo. So: all the enzyme processes are slower except for Sulfatation |
|
|
Development & Drug Distribution
|
Physicochemical properties of the drug
Cardiac output/regional blood flow Degree of protein/tissue binding Body composition: %extracellWater/intracellwater/protein/fat Premature: 60/20/10/2 [ECwater] Newborn: 40/30/20/10 [ICwater] 4mo: 30/20/20/30 [fat] adult: 15/50/20/18 [IC water] Extracellular water (decreases in proportion) Adipose tissue (prematures fat quituples to the 4mo year old then decreases) |
|
|
Sulfa drugs distribution in newborns (not prematures)
|
higher apparent volume distribution because they have more fat in proportion compared to adults;
|
|
|
Summary of differrences:
Absorption – Availability - Distribution – Metabolism – Excretion/Elimination – |
Absorption – gastric pH changes
Distribution – water and fat content change, effusions, ascites Availability - protein binding (albumin is different) Metabolism – enzymes upregulated after birth, liver fct Excretion/Elimination – kidneys mature with age Pharmacokinetics (some understanding) Ascites/effusions – increase volume of distribution; slow elimination Organ impairment – slow elimination Pharmacodynamics (little understanding) Disease can up-regulate or down-regulate receptors Was the level drawn correctly? How does the drug work? -- What numbers are important? Is the level safe? Is the level effective? |
|
|
Gentamycin in children
|
concentration dependent killer
need a peak lvl for the drug to be effective there's some residual effect that continues to kill even after the drug falls below therapeutic lvl the peak level of the drug tells us that the dose is inadequate (if it's 3..) or adequate (at 5) the trough tells us what is safe - we learn about the elimination ability How does gentamicin work? CONCENTRATION-dependent killer Post-antibiotic effect Efficacy based on Cmax/MIC – more killing with higher Cmax What does the peak tell us? Efficacy – Is the drug achieving adequate concentration to treat the infection? If peak levels are too low lower efficacy of the drug What does the trough tell us? Safety – How quickly is drug being eliminated? If trough levels are high higher risk of toxicity |
|
|
Understanding Vancomycin Levels
mechanism efficacy depends on? post antibiotic effect? |
How does vancomycin work?
Time dependent killer EBSE review: vancomycin prevents incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits into the peptidoglycan matrix; which forms the major structural component of Gram-positive cell walls. [can't be used for gram-negatives due to problem with cell membrane - except Neisseria] The binding of vancomycin to the D-Ala-D-Ala prevents the incorporation of the NAM/NAG-peptide subunits into the peptidoglycan matrix. [resistance comes from new peptides Ala-Lac or Ala-Ser] it's Bactericidal as the other cell wall ones; -TIME-dependent killer -LACKS post-antibiotic effect -Efficacy based on time that C remains > 4-5 x MIC What does the peak tell us? -Not much? Safety? Tissue penetration What does the trough tell us? -Efficacy -the nl trough lvls are in the 5 range - this MIC's are usually <1, if the lvl falls below that, it's not adequate to kill the bug |
|
|
You are caring for a 10 day old male, born at 28 weeks’ gestation. He is septic with E. coli, which is sensitive to gentamicin. He developed septic shock, with profound hypotension requiring pressor support. ATN develops secondary to this hypotension, and the infant becomes oliguric. The normal neonatal reference suggests a dosing of 4 mg/kg/dose Q24h for gentamicin. You measure a peak, and it is adequate at 7.9 (normal 5-10). You measure a trough level, and it comes back at 3 (normal <1.5). What is most appropriate step?
Lower dose at the same interval? Lower dose at a longer interval? Higher dose at the same interval? Higher dose at a shorter interval? |
Peak is adequate --> you don’t need to adjust the actual dose!
BUT Trough is high --> clearance is impaired (likely secondary to renal injury, ATN) [the interval should be adjusted - so here, we just have to wait] Therefore, hold the current dose, check another trough level in 6-12 hours. -If still >1.5, hold dose and check again in 6-12 hours -Give same absolute dose, and check trough again if the trough falls below <1.5 then give the same dose again if the peak had been low and the trough at 0.6 - then the dose wold have to be increased. the interval is kept because it's excreted normal peak high but trough 1 - so it's too high dose but interval ok peak out of range= adjust dose trough out of range= adjust the interval |
|
|
Most common heart defect in trisomy 21
|
AV canal
endocardial cushion defect |
|
|
Turners heart defect
|
pulmonary valve and aortic coarctation
|
|
|
causes of structural heart defects in neonates
|
genetic syndromes
poorly controlled maternal DM Teratogenic exposure -Li -->Ebstein’s anomaly (huge heart on CXR) -Retinoic acid --> (Transposition of the Great Arteries (TGA) [“egg on a string” by CXR], Tetralogy of Fallot (TOF) [“boot-shaped heart” on CXR], & Double Outlet Right Ventricle (DORV) -Rubella infx early in pregnancy (leads to a PDA) |
|
|
what is with most of the heart defects in utero
|
hemodynamically asymptomatic in utero
|
|
|
incidence of all CHD
|
1%
50% are significant and 50% of theses result in morbidity and mortality |
|
|
Murmurs
incidence? HD or healthy? all HD with murmur? |
Murmurs=
Turbulent blood flow and thickened valves Not all murmurs indicate heart disease ~50% of healthy children have murmurs (e.g. “functional” murmurs) Not all heart disease manifests with murmur TGA is most common “silent” CHD |
|
|
Innocent murmur
|
Stills: blood vibrates the ventricular wall - hear best at the left sternal border
Pulmonary flow murmur: from an ASD - blood being moved essentially from the LA to RA (that's not what causes the sound), flowing fast across the pulmonary valve is making the sound (vs. in a large VSD the turbulence is across the defect) Venous hum - best over clavicles; turbulent venous flow; can hear it over liver too - blood draining to IVC. Physiologic peripheral pulmonic stenosis (=obstruction at the level of the main pulmonary artery, at its bifurcation, or at the more distal branches)[Ehlers-Danlos, and Williams syndromes] |
|
|
Non-innocent murmur
list clues about it being non-innocent |
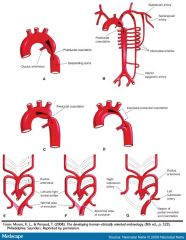
Patent ductus arteriorsus - the smaller the louder the mumur (expect to hear best over sternal border, where the septum is)
Coarctation of aorta: duct is supplying the bottom part of the body normally (get right arm and lower extremities - if the duct is placed ahead, the left arm is post ductal, so the leg and arm will both be post ductal and you won't see the difference) How to tell if it's innocent: History: growth patterns, exercise tolerance, feeding difficulty, tachypnea Physical: cyanosis, hepatomegaly, 4-extremity blood pressures (must use the right arm and a leg - you can get a false negative and miss it if the duct is placed just right - Arterial hypertension in the right arm with normal to low blood pressure in the lower extremities is classic (difference of >10mmHg). If the coarctation is situated before the left subclavian artery, asynchronous radial pulses will be detected in the right and left arms. A radial-femoral delay between the right arm and the femoral artery would be apparent, whilst no such delay would occur under left arm radial-femoral palpation. A coarctation occurring after the left subclavian artery will produce synchronous radial pulses, but radial-femoral delay will be present under palpation in either arm.) |
|
|
Most common CHD defects at birth: (decreasing order of frequency).
|
Most common CHD defects at birth: (decreasing order of frequency).
#1 VSD PDA Tetralogy of Fallot ASD Coarctation of Aorta TGA AV canal defects |
|
|
Cardiomyopathy
cause, signs, studies, interventions, outcomes |
Typically viral associated (esp. Coxsackie B)
Can be genetic Presentation (in children) usually with signs of poor cardiac output: Weak pulses Cool extremities Tachycardia Pale and/or mottled skin (can be cutus mormorada - a benign vasoinstability, but it can also be due to this heart problem) -sweating from eating Studies: Lymphocytosis/neutropenia on CBC Echocardiogram with global hypokinesis Cardiomegaly on CXR Low voltage ECG with possible arrythmia Cardiac catheterization with muscle biopsy (!!) Infants present with non-specific signs Common physical findings: Rales S3 gallop AV valve regurgitant murmur Hepatomegaly ± edema, ± JVD Interventions: Minimize metabolic and hemodynamic demands Maintain tissue perfusion (inotropic support) Diuretic and afterload reduction (lasix and milrinone) Control arrhythmias ± IV gamma globulin Outcomes: Can see complete recovery in up to 50% May need cardiac pacing, meds to control arrhythmias for months/years Cardiac transplant is an option |
|
|
Most common congenital heart defect with:
Trisomy 21 |
Trisomy 21 50 Endocardial cushion defect
|
|
|
Most common congenital heart defect with:
Trisomy 18 |
Trisomy 18 90 VSD, ASD, PDA, PS
|
|
|
Most common congenital heart defect with:
Trisomy 13 |
Trisomy 13 80 VSD, ASD, PDA,
dextrocardia |
|
|
Most common congenital heart defect with:
XO |
XO (Turner Syndrome) 20 Coarc , aortic stenosis
|
|
|
Most common congenital heart defect with:
Charge Association (coloboma, heart, choanal atresia, defects,growth retardation, genital and ear anomalies) |
80 TOF, endocardial cushion defects, VSD, ASD
|
|
|
Most common congenital heart defect with:Di George Syndrome
(Velocardiofacial syndrome) |
80% Aortic arch anomalies
|
|
|
Most common congenital heart defect with:
VACTERL (Vertebral, anal, cardiac, tracheoesophageal, radial, and or renal, limb anomalies) |
VACTERL 50 VSD
|
|
|
Most common congenital heart defect with: Marfan
|
60 Dilated or dissecting aorta,
Aortic valve regurgitation,MVP |
|
|
Most common congenital heart defect with:Williams Syndrome
|
75 Supravalvular aortic
stenosis, Peripheral pulmonic stenosis |
|
|
Most common congenital heart defect with:Infant of Diabetic Mother
|
Hypertrophic
cardiomyopathy, VSD |
|
|
Fetal Alcohol Syndrome
heart defects |
Fetal Alcohol Syndrome VSD, ASD
|
|
|
Congenital Rubella Syndrome
heart defects |
Congenital Rubella Syndrome PDA, Peripheral pulmonic
stenosis, Mitral regurgitation |
|
|
Most common intrinsic airway abnormality:
|
Tracheo-esophageal fistulae (TEF)
Results from abnormal partitioning of the foregut during the EMBRYONIC stage of lung development Incidence of 1:3000-4000 live births |
|
|
Surfactant is not produced until ...
|
Surfactant is not produced sufficiently until ~34 weeks (it starts at 26th week)
-Maternal steroids increase/accelerate production -Decreased production in IDM, certain families, Caucasian males -Surfactant is inactivated by meconium aspiration and bacterial products (term baby who aspirates meconium will likely be surfactant deficient - same with the baby with pneumonia) -number of alveoli increase from 50millino at birth to 300 million by 3-8yrs of life (good regeneration even if you have to be on a vent if you're premature - still have lot's to grow) |
|
|
The number of alveoli increase...
|
The number of alveoli increase from 50 mil at birth to ~300 mil by 3-8 years of life
|
|
|
Features of Respiratory Distress
|
Newborn
Tachypnea (resp rate > 60) Grunting Nasal flaring Cyanosis Subcostal retractions Decreased breath sounds Stridor Older children All the features that newborns have PLUS: Wheezes, rales, or ronchi Altered mental status Altered speech patterns |
|
|
Why are children more prone to respiratory distress than adults?
|
Small diameter of airways --> significantly higher airway resistance (resistance is inverse to radius to the 4th power)
Relative large size of tongue compared to size of mouth Head size in infants is proportionately larger, with less neck muscle tone --> more obstructive apnea Diaphragm is major muscle of respiration in young children --> ANY abdominal pathology can lead to respiratory compromise (paralysis, appendicitis, abdominal anything) |
|
|
Causes of respiratory distress: Newborns
Upper airway (larynx and above) vs. Lower airway (below the larynx) |
Upper airway
1) Choanal atresia -Unilateral vs. bilateral -Neonates are obligate nose breathers! -Difficulty passing suction catheter -Treatment: surgically placed stents 2) Laryngomalacia ("malacia = floppy") -Collapse of epiglottis/arytenoid cartilages -Inspiratory stridor: loudest when supine, agitated, with URIs or with feeds -Treatment is supportive (may need surgery) -Usually outgrow by 2-3 years Lower airway 1) Transient tachypnea of the newborn (TTN) 2) Respiratory distress syndrome (RDS) =Hyaline Membrane Disease (HMD) 3) TEF (VATERL and CHARGE) 4) Meconium aspiration syndrome (MAS) 5) Vascular rings 6) Pneumothorax 7) Pneumonia Not all infants/children with respiratory distress have lung disease Not all infants/children with cyanosis have heart disease So…How do you differentiate the two? Blue kid breathing comfortably = HD blue kid with breathing difficulty = lung disease |
|
|
Tracheoesophageal Fistulae
|
most common is the distal fistula and blind esophagus
Often associated with other congenital anomalies (CHARGE and VACTERL) Diagnosis made by excessive oral secretions/cyanosis, or difficulty in passing orogastric tube Treatment is surgical |
|
|
Causes of respiratory distress: Infants and Children
|
Upper airway
Laryngomalacia Tonsillar/adenoid hypertrophy Lower airway Tracheomalacia Incomplete cartilage development Common lesion in children with TEF Usually self-limited Pleural effusion Infections Croup Pneumonia Bronchiolitis Not all infants/children with respiratory distress have lung disease Not all infants/children with cyanosis have heart disease So…How do you differentiate the two? Blue kid breathing comfortably = HD blue kid with breathing difficulty = lung disease |
|
|
How important are the hormone effects?
what happens if you have too much or too little insulin? |
Too little insulin (deficient anabolism) --> intrauterine growth restriction
Too much insulin action (excessive anabolism) --> macrosomia |
|
|
fetal hyperinsulinemia causes increased growth of liver, skeletal and cardiac muscle and subcutaneous fat.
why is the cardiac muscle so important? |
they can get hypertrophic cardiomyopathies,
asymmetric interventricular septal hypertrophy --> poorely working heart --> risk for SID |
|
|
normal adult arterial O2
normal fresh term newborn: arterial O2 umbilical venous pO2 how to compesate? |
normal 90-100 for adult
50-80 newborn 20-30 in utero Hct= |
|
|
What is the transition from fetal to neonatal circulation?
most susceptible when? |
Develops between 3rd and 8th weeks
Period of highest sensitivity for malformation: 3-6½ weeks Prenatal organ of respiration RV output bypassing the lungs via PDA (R-->L) and PFO Post-natal closure of the PDA and PFO UAs obliterate and form medial umbilical ligaments UV obliterates and forms ligamentum teres hepatis DV closes and forms ligamentum venosum |
|
|
DiGeorge VCF
cardiac abnormalities |
Aortic arch problems
|
|
|
Williams syndrome
cardiac abnormalities |
pulmonary stenosis
|
|
|
Turners
cardiac abnormalities |
pulmonary valve defects and aortic arch defects (coarctation)
|
|
|
Trisomy 18
cardiac abnormalities |
VSD
|
|
|
MOST STRUCTURAL HEART DISEASE IS NOT ____
|
MOST STRUCTURAL HEART DISEASE IS NOT HEMODYNAMICALLY SIGNIFICANT IN UTERO!
|
|

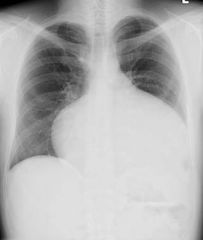
You are called to the Newborn Nursery to evaluate a 1 hour old infant with mild respiratory distress
Unremarkable pregnancy and birth history Born at 38 weeks via scheduled C-section On your arrival, vitals are normal except for a RR of 60-90 and SaO2 in room air of 82-92% Physical exam is significant for diffuse crackles on lung auscultation, but otherwise benign You obtain a CXR, and find the following: |
Transient Tachypnea of the Newborn
|
|
You are called to the Newborn Nursery to evaluate a 1 hour old infant with respiratory distress
Unremarkable pregnancy and birth history Born at 38 weeks via scheduled C-section On your arrival, vitals are normal except for a RR of 60-90 and SaO2 in room air of 79% Physical exam is significant for diffuse crackles on lung auscultation, a Grade III/VI continuous murmur over the right sternal border, and subcostal retractions You obtain a CXR, and find the following: |
Cyanotic heart disease (Ebstein’s Anomaly)
|
|
|
You are called to the Newborn Nursery to evaluate a 1 hour old infant with respiratory distress
Unremarkable pregnancy and birth history Born at 38 weeks via scheduled C-section On your arrival, vitals reveal a RR of 60-90, SaO2 in room air of 84% Physical exam is significant for a “wet” cough, drooling, and subcostal retractions You obtain a CXR, and find the following: |
Esophageal atresia with TEF
|
|
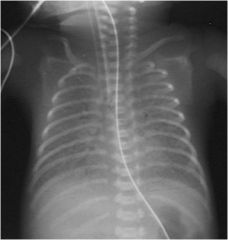
You are called to the Newborn Nursery to evaluate a 1 hour old infant with respiratory distress
Pregnancy complicated by gestational diabetes mellitus (last HgbA1c of 8.9% (normal <7%)) Born at 37 weeks via scheduled C-section On your arrival, vitals reveal a RR of 60-90, SaO2 in room air of 84% Physical exam is significant for diffuse crackles on lung exam and subcostal retractions You obtain a CXR, and find the following: |
Hyaline Membrane Disease (Surfactant Deficiency)
|
|
|
You are called to the Newborn Nursery to evaluate a 1 hour old infant with respiratory distress
Pregnancy and delivery are unremarkable Born at 39 weeks via scheduled C-section On your arrival, vitals reveal a RR of 60-90, SaO2 in room air of 84% Physical exam is significant for severe subcostal retractions You are unable to pass a 5 Fr suction catheter through either side of the nose |

Choanal atresia
|
|
|
You are evaluating a 9 month old girl in your office with chief complaint of cough and respiratory distress
She has had URI symptoms for 3 days with lots of secretions Exam reveals a RR of 50-70, and breathing that sounds like: |
Croup
|
|
|
Normal XY Puberty: Sequence
|
Adrenarche
Pubarche (pubic/axillary hair) Gonadarche (testicular enlargement) Penile enlargement Height spurt |
|
|
Normal XY Puberty: Timing
|
Adrenarche/Pubarche:
Difficult to pinpoint start; begins several years before outward signs noted Increased levels of DHEA Is gonadotropin-independent Testicular enlargement: Is gonadotropin-dependent (controlled by hypothalamic- pituitary-gonadal axis) GnRH LH Testosterone Starts at 11½ years (range 9½ to 13½ yrs.) Tanner 1 Testes : long axis < 2.5 cm Penile enlargement: Begins about 1 yr. after testicular enlargement (at about 10½ to 14½ yrs.) Height spurt: peak at 14 yrs. Limbs accelerate before trunk Distal limbs accelerate before proximal limbs Average span of XY puberty is 3.5 yrs |
|
|
Normal XX Puberty: Sequence
|
Adrenarche
Pubarche Gonadarche (activation of ovaries) Thelarche (breast development) Height spurt Menarche (1st menses) |
|
|
Normal XX Puberty: Timing
|
Adrenarche
Gonadarche Thelarche (tanner 2 breast nub) Is gonadotropin-dependent GnRH FSH Estrogen 6½ to 12 yrs age Full breast dev takes about 4 yrs Height spurt Increased velocity begins about 1 yr. after thelarche starts Menarche (1st menses) Preceded by peak height velocity by ~6 mos. Usually during Tanner 4 breast Most girls only grow about 1-2 inches after menarche Breast buds to menarche ~ 2.3 yrs. Average span of XX puberty ~ 4.2 yrs. |
|
|
Gonadarche contrasted with adrenarche:
|
Gonadarche should be contrasted with adrenarche. Gonadarche indicates that true central puberty has begun, while adrenarche is an independent maturational process only loosely associated with complete puberty.
Gonadarche refers to the earliest gonadal changes of puberty.[1] In response to pituitary gonadotropins, the ovaries in girls and the testes in boys begin to grow and increase the production of the sex steroids, especially estradiol and testosterone. * In boys, testicular enlargement is the first physical sign of gonadarche, and usually of puberty. * In girls, ovarian growth cannot be directly seen, so thelarche and growth acceleration are usually the first evidence of gonadarche. Adrenarche refers to a stage of maturation of the cortex of the human adrenal glands. It typically occurs between ages 6 and 10 years and involves both structural and functional changes. Adrenarche is a process related to puberty but distinct from hypothalamic-pituitary-gonadal maturation and function the independence of adrenarche and gonadal puberty is apparent in children with atypical or abnormal development, when one process may occur without the other. For instance, adrenarche does not occur in many girls with Addison's disease, who will continue to have minimal pubic hair as puberty progresses. Conversely, girls with Turner syndrome will have normal adrenarche and normal pubic hair development, but true gonadal puberty never occurs because their ovaries are defective. |
|
|
Delayed Puberty definition
|
No sign of beginning puberty by:
13 yrs. in girls 14 yrs. in boys |
|
|
Differential for Pubertal Delay
|
Variation of normal
Hypogonadotropic hypogonadism Hypergonadotropic hypogonadism Variation of normal /familial Hypogonadotropic (secondary) hypogonadism CNS abnormality (ex. tumor, panhypopituitarism, trauma) Malnutrition Drugs (marijuana) Endocrine (hypothyroidism, hyperprolactinemia, diabetes mellitus, Cushing’s Dz) Isolated gonadotropin deficiency Syndromic (Prader-Willi Syndrome) Syndrome Chronic disease Hypergonadotropic (primary) hypogonadism Chromosomal abnormality (Klinefelter Syndrome (XXY), Turner’s Syndrome (XO)) Cryptochidism/anorchia Pure gonadal dysgenesis Trauma Post-infectious (mumps) Treatment for neoplasia |
|
|
Hypogonadism: Timing
|
If occurs prenatally --> Ambiguous genitalia
If occurs before puberty --> Delayed/failed puberty Occurs after puberty: XY—sexual dysfunction XX—secondary amenorrhea |
|
|
Precocious Puberty define
|
Secondary sexual development before age:
8 years in XX 9 years in XY |
|
|
Differential for Precocious Puberty
|
Gonadotropin-dependent PP (Central PP)
Constitutional/familial CNS anomalies (tumors, hydrocephalus, trauma, inflammation) Previous androgen exposure (poorly controlled CAH, McCune-Albright) Idiopathic (up to 90%, 20X more common in ♀) Gonadotropin-independent PP (Peripheral PP) Ovarian cysts/tumors (↑ estrogen, present with vaginal bleeding) Leydig cell tumors / premature activation (↑ testosterone, present with asymmetric testicular enlargement) Adrenal tumors Pituitary GnRH-secreting tumors McCune-Albright Syndrome Incomplete PP Early development of secondary sexual characteristics Isolated premature adrenarche or thelarche Usually a variant of normal Bone age and height velocity are NOT accelerated Warrant close follow up, as ~20% can go on to develop true GDPP |
|
|
Central Precocious Puberty
|
Early maturation of the ENTIRE hypothalamic-pituitary-gonadal axis
Results in full spectrum of physical & hormonal changes of puberty (rather than isolated or mixed) IN THE CORRECT SEQUENCE AND PACE The Initial Evaluation: History (When did the changes begin? Exposures? Other diseases/symptoms?) Physical exam (Height, weight, arm span, evaluation for papilledema, visual fields, dermatologic findings, PUBERTAL STAGING) Bone age determination; evaluate further if: bone age > chronological age Normal bone age, but evidence of PP NOTE: With normal bone age, unlikely to have Gn-dependent PP NOTE: With normal bone age and evidence of incomplete PP, close follow up is appropriate without further workup unless other signs develop Further evaluation: LH serum (basal and after GnRH agonist) A)“Pubertal” LH level with ↑ after GnRH agonist --> GnRH-dependent PP =CNS imaging (MRI) Thyroid function tests Idiopathic GnRH-dependent PP is diagnosis of exclusion B) “Prepubertal” LH and no response to GnRH agonist--GnRH-independent PP =Serum hormone levels: Testosterone Estradiol Cortisol DHEAS 17-OH-Progesterone Abdominal/Testicular US |
|
|
Treatment for PP
|
Dependent on eventual cause of PP
For GnRH-dependent PP, sustained use of GnRH agonists Pituitary responds to pulsatile GnRH, and with sustained GnRH, there is negative feedback on pituitary to reduce production of gonadotropins For GnRH-independent PP, surgery/radiation/chemotherapy to address underlying pathology For incomplete PP, close follow up unless progression to true central PP |
|
|
male development: what is gonadotropin dependent? what is independent?
|
Adrenarche/Pubarche:
Is gonadotropin-independent Testicular enlargement: Is gonadotropin-dependent (controlled by hypothalamic- pituitary-gonadal axis) |
|
|
testes <2.5cm
|
Tanner stage 1
|
|
|
height spurt peak for guys - what's a marker for having started?
|
arms and legs grow fast first
arm-span < height = haven't started yet peak: 14ys |
|
|
female development: what is gonadotropin dependent? what is independent?
|
Thelarche
Is gonadotropin-dependent |
|
|
Menarche usually happens at what Tanner stage ?
|
4
6mo later they have finished height growth |
|
|
if bone age is normal - is it more likely to be central or peripheral PP
|
NOTE: With normal bone age, unlikely to have Gn-dependent PP
NOTE: With normal bone age and evidence of incomplete PP, close follow up is appropriate without further workup unless other signs develop |
|
|
treatment for GnRH-dependent PP
|
For GnRH-dependent PP, sustained use of GnRH agonists
this works because: Pituitary responds to pulsatile GnRH, and with sustained GnRH, there is negative feedback on pituitary to reduce production of gonadotropins |
|
|
Case:
15 year old ♂ Exam: Weight 25% & Height at 65% for age No deepening of voice Testicular length 2.8cm (Tanner 2) Sparse, fine hair around base of penis, no axillary hair (Tanner 2) Penile exam consistent with Tanner 1 Xray for bone age: consistent with 12yrs. Parents’ pubertal histories: father was a “late bloomer” Charles’ arm span is < height Normal neurologic exam Serum LH levels are in pre-pubertal range Charles most likely has what? |
Keys: Age > 14yrs.
Not obese, arm span not > Ht. [so not syndromic] No indications of testosterone effect [so not taking drugs…] Delayed bone age Dad “matured late” Normal neuro exam Delayed puberty (variation of normal) [NOT Prader-Willi Syndrome Kallman Syndrome,Hypogonadotropic hypogonadism from craniopharyngioma] |
|
|
½ year old Caucasian ♀
Mom brought Sally in because “she’s growing where she shouldn’t” Exam shows breast buds bilaterally Weight 45% and Height 60% for age Fine, dark hair over mons veneris No enlargement of the clitoris No facial hair Bone age 7 yrs. Dx? LH in Tanner 2 pubertal range Estrogen in Tanner 2 pubertal range DHEAs in Tanner 2-3 range Thyroid tests normal |
Keys: Age < 7yrs.
Beginning breast development (thelarche) Signs of adrenarche (fine, dark hair) Slightly increased bone age Pubertal level LH, estrogen, DHEAs Isosexual Normal pubertal progression Pelvic U/S shows Tanner 2 ovarian size Head MRI normal Appropriate LH response to GnRH Dx: Idiopathic Central Precocious Puberty everything else is excluded |
|
|
8½ year old ♂
Exam: Weight 40%, Height 90% Few fine, dark hairs at base of penis Testicular size Tanner 2-3 No breast enlargement Bone age 10 yrs. LH levels prepubertal High testosterone |
Measure serum DHEAs and 17-OH-progesterone
Normal for chronologic age Go back and carefully examine Ben again—the right testis has a slightly different consistency, and is ? larger Leydig cell tumor |
|
|
10 year old ♀
Weight 50%, Ht 75% No facial hair Breasts: small mounds, areolas enlarged Dark, coarse, curly hair spreading slightly over mons veneris Clitoris not enlarged No masses in labia or inguinal area Mom’s age at menarche was 11 yrs. Do we need to do labs or a bone age? Is this normal, isosexual pubertal sequence? |
10 yrs. (not too young for puberty)
?Beginning increase in Ht velocity No indications of contrasexuality Breasts about Tanner 3 Pubic hair Tanner 3 (consistent) Mom’s menarche was at 1 yr. older so it's Normal Female Pubertal Development! |
|
|
You are called to attend the delivery of a infant at 28 weeks’ gestation
Mother is a 24 year old primigravida who presented with vaginal bleeding TRUE OR FALSE: Each of the following can present with vaginal bleeding: Placenta previa Placenta percreta Placental abruption Rupture of velamentous cord insertion |
all True
|
|
|
During labor, the mother develops a temperature to 101°F, and the OB notes significant uterine tenderness
Maternal heart rate of 120 and fetal heart rate of 190-210 Infant is delivered without incident, and admitted to the NICU on minimal respiratory support |
Chorio
|
|
|
Now DOL 10, feeding well until midnight
Found at 1am with RR 80, labored; pale; HR 185 When you arrive, the nurse is distraught and insists that the baby was fine at the midnight feed “But now he’s so pale and lethargic,” with significant respiratory distress What is your differential? |
TTN?
Pneumonia? HMD (surfactant deficiency)? Could this be choanal atresia? MAS? Neonatal respiratory distress: Choanal atresia (DOL 1) TTN or HMD (DOL 1) MAS (DOL 1) TEF (DOL 1-4) Infection (anytime) Congenital heart disease (DOL 1-10) [child in distress can have a duct dependent lesion] |
|
|
Infant respiratory distress:
Ddx |
Laryngo/tracheomalacia
Infection Bacterial Viral (bronchiolitis, croup) CHD Metabolic defect |
|
|
Childhood respiratory distress
ddx |
Hypertrophy of Tonsils/Adenoids
Infection Bacterial Viral (bronchiolitis, croup) Laryngo/tracheomalacia |
|
|
TRUE OR FALSE?
Reasons children are more prone to respiratory distress : Less airway resistance than adults Tongue larger relative to mouth in child The most important muscle of respiration in the child is the diaphragm Obstructive apnea more common due to big head, low tone |
F: higher resistance than in adults
T T T |
|
|
Respirations are “gasping” in nature
Lungs clear Murmur: machinery-like, continuous murmur with bounding pulses at the left sternal border Mottled skin, pale The infant’s skull is misshapen: AP length is much shorter than transverse length You note that James has upslanting palpebral fissures with epicanthal folds, a large tongue, and bilateral single palmar creases |
Down syndrome
|
|
|
Which of the following are generally innocent murmurs?
Low pitched, vibratory systolic murmur along the left sternal border Soft, blowing systolic murmur radiating to the back and both axillae A soft, blowing systolic murmur at the LUSB |
Stills
Pulmonary branch stenosis Pulmonic flow (related to ASD would be not innocent – BUT if you only hear this if the person is laying then it’s not an ASD but just increased flow across the pulmonic valve) |
|
|
If James has CHD, which of the following is most associated with the spectrum of findings on his physical examination:
TGA Complete AV canal Coarctation Dilated cardiomyopathy |
B - the complete AV canal
|
|
|
You ask for an electrolyte panel and glucose ( normal except for WBG of 339)
You ask for a CBC Hct 38%, platelets 314K (“Not bad”, you think) WBC 4.7 (12segs, 11bands, 74lymphs) Is the CBC a reflection of James’ immature immune system? could be Which statements about James’ immune system are true? a) His PMNs aren’t good at phagocytizing gram negative bacilli. b) His complement levels are 150% that of a 7-year-old. c) He is deficient in maternal IgG. d) His CD4/CD8 is less than that of an adult. |
a) yes
b) false they're 1/2 c) the IgG is transferred in the 3rd trimester --> a 28wk gestation will be deficient d) true |
|
|
Group B strep antibiotic
|
ampicillin
|
|
|
Gram negative rods
|
Cefotaxime
|
|
|
decreased blood flow to the placenta
|
drugs.... hypoperfusion of the fetus too
|
|
|
causes of DIC
|
STOP making New Thrombi
|
|
|
neonatal causes of Meningitis
|
Listeria, E.coli, groupB strep
|
|
|
Which of the following are true in James:
Lower drug clearance than in term neonates Kidney function is better in term neonates Protein binding of drug is less in premature Half-life of gentamicin is longer in premature than term |
all True
|
|
|
James’ gent trough is 4.3 (normal: <1.5)
What do you do next: Give a lower dose at a longer interval Give a higher dose at a longer interval Give a lower dose at a shorter interval Hold the gentamicin dose and repeat a level in 12 hrs. |
D
|
|
|
Overwhelming septic shock (“warm shock”) with multiorgan failure, DIC & seizures due to late-onset GBS Disease
Is this overwhelming infection an indication that James has an immune deficiency? |
“Classic” presentation of immune disorder:
Male Newborn to 5yrs. Increased “usual” infections Eczema Autoimmune disease |
|
|
True of False?
a)Probably not Chronic Granulomatous Disease because his leukocyte count is not normal or high b)If the lab reported giant granules in his PMNs, you’d worry about Chediak-Higashi Syndrome |
True
True |

