![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
Put in order of boiling points: ROOH, ROH, ROR |
ROOH>ROH>ROR First of all hydrogen bonding. Second 2 molar equivalents of hydrogen bonding in ROOH. |
|
|
Which is more acidic, alcohols (alkoxides) or carboxylic acids? Why? |
Carboxylic acids because the carboxylate anion delocalises the electron, making it more stable than alkoxides. |
|
|
Describe the solubility of carboxylic acids (pka 4.19) and phenol's (pka 10) in aqueous hydrogen-carbonate (a base, conj acid pka = 6.38) |
Carboxylic acid is stronger than the conjugate acid of hydrogen carbonate, so will dissolve. (lower pka value) Phenol is a weaker acid than the conjugate acid of hydrogen carbonate, so will not dissolve. |
|
|
Which is more reactive between acyl chloride, ester, amide and acid anhydride during nucleophilic addition? |
acyl chloride> acid anhydride > esters > amides Why? because electron withdrawing groups (eg Cl) makes the carbonyl carbon more positive, so more tendency for Nu- to attack. OR, and NH2 are electron donating groups and will make the carbonyl carbon less positive, and also forming a third resonace form, making the carbon LESS positive. acid anhydride (COORO) is more reactive than esters (COOR) because it does not form the third resonance form (due to conjugation). |
|
|
How do you prepare acyl chlorides? hint, use carboxylic acid. |
react with SOCl2 acyl chloride + HCl + SO2 |
|
|
how to form amide from acyl chloride? |
react with an amine (RNH2) |
|
|
How to form carboxylic acid from acyl chloride? |
React with H2O |
|
|
How to form ester from acyl chloride? |
react with alcohol (ROH) |
|
|
How to form annhydride from acyl chloride? what types? |
Add carboxylic acid (R*CO2H) forms O=C-OCOR* Note, symmetrical and unsymmetrical anhydrides form. |
|
|
Other than from an acyl chloride, how do you prepare acid anhydrides? What type? |
add P2O5 , a dehydrating agent. WHILE adding 2 mol equivalents of carboxylic acid!!!! It forms symmetrical anhydrides + H2O |
|
|
Using anhydrides, how do you form... 1. ester 2. amide 3. carboxylic acid? |
1. react with alcohol (ROH) 2. react with amine (RNH2) 3. react with H2O |
|
|
REMINDER of how nucleophilic additions to caboxylic derivatives look like!!!!!!!!!!!! |
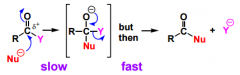
|
|
|
using esters, how to form amide? |
react with amine, not byproduct of alcohol forms. |
|
|
what are byproducts of .... ester reactions acyl chloride reactions anhydride reactions |
ester = alcohols acyl chloride = HCl anhydride = carboxylic acid Just remember HCOY, (a carbonyl group with Y, which is Cl, OR, etc) think of Y coming off then adding a H to it, eg Y=OR (ester) OR -> ROH, alcohol! |
|
|
esters form different products depending on if it is hydrolised in acid or base. Reagents? Products/ |
base = (OH-/H2O) and forms alcohol + carboxylate anion acid = (H2O, H3O+) and forms alcohol + carboxylic acid |
|
|
what are the mechanisms of acid and base catalysed hydrolysis of esters? |
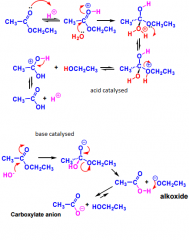
|
|
|
How does longer alkyl chain affect carboxilic acids? What about tertiary vs secondary vs primary carboxylic acid? |
decreasing acidity (if electron donating...) If tertiary, and electron withdrawing groups are attached, carboxylic acid more acidic, as it is more likely to donate proton. |
|
|
How to form carboxylic acid from grignard reagent? |
1. CO2
2. H3O+ |
|
|
How to form primary secondary or tertiary amide? |
NH3 for primary RNH2 for secondary R2NH for tertiary NOTE tertiary amines dont react. |
|
|
What is needed for Amides to undergo hydrolysis? |
STRONG aqueous base or acid. eg 1. H2SO4 2. OH- To form amine |
|
|
What are quaternary ammonium salts? |
N bonded to 4 groups, having a positive charge. |
|
|
What is aliphatic and armoatic amines? |
aliphatic = amine group bonded to another carbon. aromatic = amine group dircetly on benzene ring. |
|
|
RNH, as R (say it is CH3 for now) increases, what happens to basicity? |
it increases due to more electron donating, less likely to donate proton. However tertiary amine is LESS basic, due to steric effect. |
|
|
Compare strength of aryl and alkyl amines? What about electron donating vs electron withdrawing on aryl amines (para)? |
aryl amines weaker bases, as delocalisation of electrons make it less available to act as bast. More electron withdrawing, LESS BASIC. EWG Ar-NH3 < EDG Ar-NH3 < Ar-NH3 < Aliphatic NH3 in terms of basicity. |
|
|
how to form amine from alkyl halide? |
nucleophilic substitution of NH3 or RNH2 and so on. |
|
|
How to convert amide (or nitrile, imine, oxime) into amine? |
reduction, using LiAlH4, followed by H2O. Remember that N substituted amides/imines secondary forms, and NN substituted tertiary amines form. |

