![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
|
What is Biomolecule ? |
All the carbon compounds thay we get from living tissues are called Biomolecules. |
|
|
Is this True: All the elements present in a sample of earth’s crust are also present in a sample of living tissue. |
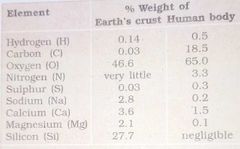
Yes , but thier relative abundance varies. |
|
|
How to Analyze chemical composition ? |
Have both Organic and Inorganic compounds... Now lets check both one by one |
|
|
For Organic Compound: (chemical analysis) |

|
|
|
How to Identify a particular compound from all other compounds ? |
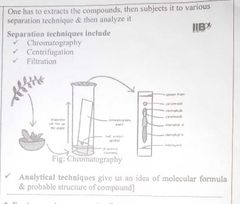
|
|
|
For Inorganic Compounds:(Destructive technique) |

|
|
|
▶CARBOHYDRATES /SACCHARIDES |
. |
|
|
CARBOHYDRATES/SACCHARIDES |
[🔰CHE]defined as optically active polyhydroxy aldehydes or ketones or the compounds which produce such units on hydrolysis. |
|
|
General Points
|
▶General Formula : Cn(H2O)n n = no. of monosaccharide links ▶Compounds of C,H,O where ratio of H & O is 2:1 ▶In body,55% of energy is available from carbohydrate diet. ▶Each Saccharide has basic unit sugar. ▶Carbohydrates are produced in Chlorophyll containing cells during photosysthesis. therefore, constitute major part of the dry weight of plants (upto 80%).
|
|
|
🔴[🔰CHE]▶Give some compounds following general formula of carbohydrates, but aren't carbohydrates ▶Give some compounds not following general formula of carbohydrates, but are carbohydrates |
▶☑1.Formaldehyde (HCHO) ☑2.Lactic acid (CH3CHOH.COOH)
▶☑1. Rhamnose (C6H12O5) (Deriavative of Glucose) (Deriavative of Glucose) ☑3. Deoxyribose (C6H10O4) |
|
|
[🔰CHE]▶Classificationof carbohydrates on basis of Reducing or non reducing sugars |
. |
|
|
▶What is meant by Anomeric Carbon ? |
▶An anomeric carbon is the first stereocenter of the molecule.
▶ If that stereocenter has an OH group coming off of it then it is a reducing sugar. ▶ Here both anomeric carbons are involved in the bond, neither one has an OH group, so it is non reducing sugar. |
|
|
▶What are Reducing Sugars ? |
▶A reducing sugar is a sugar that has a free aldehyde or ketone that can act as a reducing agent. ▶This can reduce Cu2+ of Benedicts or Fehlings solution to cuprous ions. Blue colour of solution changes to brick red. ▶All monosaccharides are reducing sugars.
▶Disaccharides may be reducing or not.
▶Maltose is a combination of two glucose molecules. They are combined using the first carbon (the anomeric carbon) from one of the glucose molecules and the fourth carbon from the other glucose molecule. Since the second glucose molecule still has the anomeric carbon available with the OH group, it is still able to act as a reducing sugar.
|
|
|
▶What are Non Reducing Sugars ? |
▶A non-reducing sugar does not have a free aldehyde or ketone, so it cannot act as a reducing agent ▶Do not reduce Cu2+ ios of Fehling solution..... ▶The main non-reducing sugar is sucrose, or more commonly known as table sugar. ▶Sucrose is a glucose carbon connected at the anomeric carbon to an anomeric carbon on a fructose |
|
|
▶On the basis of number of sugar units obtained upon hydrolysis, gives the Classification as : |
▶1.Monosaccharide - ✔1 unit (monomer) ✔ Cannot be hydrolysed further
▶2. Oligosaccharides : ✔Yield two to ten monosaccharide units on hydrolysis
▶3.Polysaccharides: ✔Yield a large number of monosaccharide units on hydrolysis |
|
|
Monosaccharide |
. |
|
|
▶General Points |
▶Crystalline,soluble in water ▶Acts as building blocks for the formation of complex carbohydrates ( cellulose,starch,and glycogen) |
|
|
▶Clasification Based on No. of carbon atoms ( 5 types ) |
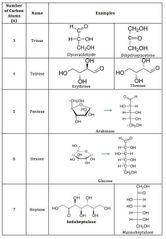
|
|
|
▶▶▶▶▶▶▶▶▶ |
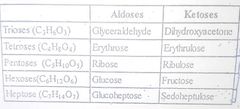
▶Pentoses (C5 H1O O5), e.g., Ribose, Xylose, Deoxyribose,Arabinose, Ribulose. Deoxyribose is an exception because it has a formula of C5 H1O O4. ▶Hexoses , e.g., Glucose, Fructose, Galactose,Mannose. Glucose has aldehyde group at carbon 1 While fructose has ketone at carbon 2. |
|
|
▶Structure of Ribose ,Ribulose, Glucose, Galactose,Fructose
▶Few Modifications and Points |
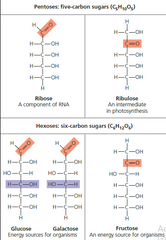
1)Deoxygenation (Removal of O at 2nd Carbon) of ribose produce deoxyribose.
2)Modified Sugars are also called as : Derived Sugars ex. Ribose & deoxyribose, glucoronic acid,glucosamine,mannitol. |
|
|
▶Ribose ➡➡➡Deoxyribose |
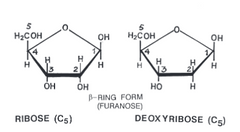
1)Deoxygenation(Removal of O at 2nd Carbon) of ribose produce deoxyribose. |
|
|
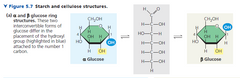
▶Two ring forms for glucose are called alpha (α) and beta (β) |
|
|
|
▶About Pyranose ring(Glucose) and Furanose ring(Fructose) |
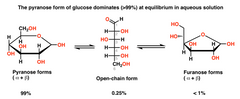
▶Pentoses and hexoses exist in both open chains as well as ring forms. There are twotypes of ring forms, pyranose and furanose
▶Pyranose has hexagon structure with five carbons and one oxygen. Furanose has a pentagon structure with four carbons andone oxygen. ▶Both pyranose and furanose types are further distinguished into alpha and beta types. In alpha form the hydroxyl group nearer the oxygen atom of the ring is written below while InBeta form it is written above. ▶Many monosaccharides have asymmetric carbons and are ableto rotate polarised light to right side (D, or dcxtrorotatory, +) or left side (L, orlaevorotatory, —). Amongst sugars D—isomers are more common. |
|
|
▶Glycosidic Bond |
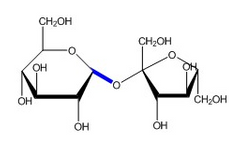
▶Glycosidic bonds are covalent chemical bonds that hold together a glycoside. ▶A glycoside is simply a ring-shaped sugar molecule that is attached to another molecule ▶. Glycosidic bonds can either be O-linked or N-linked. ▶ Example : Sucrose is the sugar , It is composed of two sugar units, a glucose (left) and a fructose (right), linked by a glycosidic bond. |
|
|
▶How Glycosidic Bonds are formed ?? |
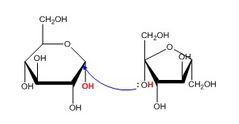
▶A glycosidic bond forms by a condensation reaction, which means that one water molecule is produced during formation of a glycoside. (Dehydration Synthesis)
The reverse reaction, the breakage of a glycosidic bond, is a hydrolysis reaction. One water molecule is used up in the reverse reaction. ➡The condensation reaction occurs when an alcohol group, or OH, from a molecule attacks the anomeric carbon of a sugar. ➡When the alcohol attacks the anomeric carbon, the OH group bonded to that carbon is replaced by the O of the alcohol, and the H of the alcohol is removed. As you can see, both an H and an OH (shown in red) are removed from the original molecules during the reaction. Together they make H2O. ▶ Ether formed which is relatively unreactive compared to other chemical groups, such as alcohols. Therefore, glycosides tend to be more stable than free sugars. |
|
|
▶Condensation Reaction / Dehydration Reaction |
▶A chemical reaction in which two molecules combine and form a larger molecule, leaving behind a smaller molecule (for many reactions this small molecule is water)
|
|
|
▶Formation of Glucose and Fructose from Sucrose |
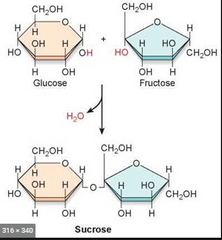
|
|
|
▶Glucose |

|
|
|
▶Fructose |

|
|
|
▶Oligosaccharides |
▶Oligo(Few sugar unit , min:2 & max:9) |
|
|
▶Classification of Oligosaccharides |
▶Disaccharides (e. g sucrose, lactose), ▶Trisaccharides (e.g., rafflnose) ▶Tetrasaccharides (e.g., stachyose), ▶Pentasaccharides, Hexasaceharidcs, Heptasaccharidcs, etc. |
|
|
▶Disaccharide |
▶Smallest & Commonest Type ▶Ex. ➡Sucrose ( non reducing sugar) ➡Maltose (reducing sugar) ➡Lactose (reducing sugar) |
|
|
▶Sucrose |
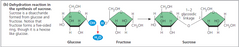
▶Obtained from Sugar cane crop - Saccaharum officinarium ▶Prepared by condensation of one glucose molecule and one fructose molecule. Glycosidic bond between C-1 of glucose and C-2 of fructose. ( alpha-1,2 linkage) ▶In sucrose, fructose occurs in the furanose (pentagon) form while glucose is in pyranose(hexagon) state. ▶Sucrose is the storage carbohydrate of Sugarcane and Sugarbeet. |
|
|
▶Maltose |
. |

