![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
923 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
The chief taxonomic category. This book recognizes six ________: Archaea, Bacteria, Protista, Fungi, Animalia, and Plantae. |
1. Kingdoms (16) |
|
|
|
A group of prokaryotes that are among the most primitive still in existence, characterized by the absence of peptidoglycan in their cell walls, a feature that distinguishes them from bacteria. This kingdom of prokaryotes (the simplest of cells that do not have nuclei) includes this methanogen (picture in book), which manufactures methane as a result of its metabolic activity. |
1. Archaea Kingdom (16)
|
|
|
|
The most common type of prokaryotic organism. Cell walls contain peptidoglycan. Play many important ecological roles. This group is the second of the two prokaryotic kingdoms. Show here (image in book) are purple sulfur bacteria, which are able to convert light energy into chemical energy. |
1. Bacteria Kingdom (16)
|
|
|
|
Includes unicellular eukaryotic organisms and some multicellular lines derived from them. Most of the unicellular eukaryotes (those whose sells contain a nucleus) are grouped into this kingdom, and so are the multicellular algae pictured here (image in book).
|
1. Protista Kingdom (16)
|
|
|
|
This kingdom contains nonphotosynthetic organisms, mostly multicellular, that digest their food externally, such as these mushrooms (picture in book).
|
1. Fungi Kingdom (16)
|
|
|
|
This kingdom contains photosynthetic multicellular organisms that are terrestrial, such as the flowering plant pictured here (image in book).
|
1. Plantae Kingdom (16)
|
|
|
|
Organisms in this kingdom are nonphotosynthetic multicellular organisms that digest their food internally, such as this ram (picture in book).
|
1. Animalia Kingdom (16)
|
|
|
|
All living things are composed of one or more cells. A cell is a tiny compartment with a thin covering called a membrane. Some cells have simple interiors, while others are complexly organized, but all are able to grow and reproduce. Many organisms possess only a single cell; your body contains from 10 – 100 trillion cells (depending on how big you are).
|
1. Cellular Organization (17)
|
|
|
|
The process by which all living things assimilate energy and use it to grow. The transfer of energy from one form to another in cells is an example of __________.
|
1. Metabolism (17)
|
|
|
|
The maintaining of a relatively stable internal physiological environment in an organism or steady-state equilibrium in a population or ecosystem.
|
1. Homeostasis (17)
|
|
|
|
All living things grow and reproduce. Bacteria increase in size and simply split in two as often as every 15 minutes, while more complex organisms grow by increasing the number of cells and reproduce sexually.
|
1. Growth and Reproduction (17)
|
|
|
|
The transmission of characteristics from parent to offspring.
|
1. Heredity (17)
|
|
|
|
At the level or position of the most basic structural and functional units of the human body; cells are organized into five levels of complexity (atoms, molecules, macromolecules, organelles, and cells).
|
1. Cellular Level (18)
|
|
|
|
A core (nucleus) of protons and neutrons surrounded by an orbiting cloud of electrons. The chemical behavior of an ____ is largely determined by the distribution of its electrons, particularly the number of electrons in its outermost level.
|
1. Atoms (18)
|
|
|
|
The smallest unit of a compound that displays the properties of that compound.
|
1. Molecules (18)
|
|
|
|
An extremely large molecule. Refers specifically to carbohydrates, lipids, proteins, and nucleic acids.
|
1. Macromolecules (18)
|
|
|
|
A specialized compartment of a cell. Mitochondria are __________.
|
1. Organelles (18)
|
|
|
|
The smallest unit of life. The basic organizational unit of all organisms. Composed of a nuclear region containing the herediary apparatus within a larger volume called the cytoplasm bounded by a lipid membrane.
|
1. Cells (18)
|
|
|
|
Cells are organized into four levels of complexity (tissues, organs, organ systems, and organism).
|
1. Organismal Level (18)
|
|
|
|
A group of similar cells organized into a structural and functional unit.
|
1. Tissues (18)
|
|
|
|
A complex body structure composed of several different kinds of tissue grouped together in a structural and functional unit.
|
1. Organs (19)
|
|
|
|
A group of organs that function together to carry out the principal activities of the body.
|
1. Organ Systems (19)
|
|
|
|
Any individual living creature, either unicellular or multicellular.
|
1. Organism (19)
|
|
|
|
Organisms are organized into four hierarchical levels within the living world (population, species, community, and ecosystem).
|
1. Populational Level (19)
|
|
|
|
Any group of individuals of a single species, occupying a given area at the same time.
|
1. Population (19)
|
|
|
|
A group of interbreeding organisms that are reproductively isolated from all other such groups; a taxonomic unit ranking below a genus and designated by a two-part scientific name consisting of its genus and the species name.
|
1. Species (19)
|
|
|
|
The populations of different species that live together and interact in a particular place.
|
1. Community (19)
|
|
|
|
A community together with the nonliving factors with which it interacts.
|
1. Ecosystem (19)
|
|
|
|
Novel properties in the hierarchy of life that were not present at the simpler levels of organization.
|
1. Emergent Properties (19)
|
|
|
|
The differential reproduction of genotypes caused by factors in the environment. Leads to evolutionary change.
|
1. Natural Selection (20)
|
|
|
|
The breeding of plants and animals to produce desirable traits. Organisms with the desired traits, such as size or taste, are artificially mated or cross-pollinated with organisms with similar desired traits.
|
1. Artificial Selection (20)
|
|
|
|
The condition in which two or more dissimilar organisms live together in close association; includes parasitism, commensalism, and mutualism.
|
1. Symbiosis (20)
|
|
|
|
Making individual decisions by applying a “guide” of accepted general principles. A logical process in which a conclusion drawn from a set of premises contains no more information than the premises taken collectively. All dogs are animals; this is a dog; therefore, this is an animal: The truth of the conclusion is dependent only on the method.
|
1. Deductive Reasoning (22)
|
|
|
|
Through observation, scientists look at the world to understand how it works and determine the principles that govern our physical world. Reasoning in which the premises seek to supply strong evidence for (not absolute proof of) the truth of the conclusion.
|
1. Inductive Reasoning (22)
|
|
|
|
Any of several simple gaseous compounds that contain carbon, chlorine, fluorine, and sometimes hydrogen, that are used as refrigerants, cleaning solvents, and aerosol propellants and in the manufacture of plastic foams, and that are believed to be a major cause of stratospheric ozone depletion.
|
1. Chlorofluorocarbons (CFCs) (23)
|
|
|
|
A proposal that might be true. No __________ is ever proven correct. All __________ are provisional – proposals that are retained for the time being as useful but that may be rejected in the future if found to be inconsistent with new information. A __________ that stands the test of time – often tested and never rejected – is called a theory.
|
1. Hypothesis (24)
|
|
|
|
The test of a hypothesis. An __________ that tests one or more alternative hypotheses and those that are demonstrated to be inconsistent with experimental observation are rejected.
|
1. Experiment (24)
|
|
|
|
The key to any successful scientific investigation is careful ___________. The action or process of _________ something or someone carefully or in order to gain information.
|
1. Observation (24)
|
|
|
|
Formed when scientists have more than one guess about what they observe; (in the statistical testing of a __________) the __________ to be accepted if the null __________ is rejected.
|
1. Alternative Hypotheses (25)
|
|
|
|
What you expect to happen if a hypothesis is true.
|
1. Predictions (25)
|
|
|
|
Attempting to verify the hypothesis’ predictions.
|
1. Testing (25)
|
|
|
|
A group or individual used as a standard of comparison for checking the results of a survey or experiment.
|
1. Controls (25)
|
|
|
|
Any factor that influences a process. In evaluating alternative hypotheses about one ________, all other _________ are held constant so that the investigator is not misled or confused by other influences.
|
1. Variable (25)
|
|
|
|
An __________ where all subjects involved are treated exactly the same except for one deviation.
|
1. Control Experiment (25)
|
|
|
|
A hypothesis that has been tested and not rejected is tentatively accepted.
|
1. Conclusion (25)
|
|
|
|
A well-tested hypothesis supported by a great deal of evidence.
|
1. Theory (25)
|
|
|
|
A method of procedure that has characterized natural science since the 17th century, consisting in systematic observation, measurement, and experiment, and the formulation, testing, and modification of hypotheses.
|
1. Scientific Method (26)
|
|
|
|
The foundation for understanding the reproduction and growth of all organisms. The ideas that all living matter consists of cells, cells are the structural and functional units of life, and all cells come from preexisting cells.
|
1. Cell Theory (28)
|
|
|
|
The basic storage vehicle or central plan of heredity information. It is stored as a sequence of nucleotides in a linear nucleotide polymer. Two of the polymers wind around each other like the outside and inside rails of a circular staircase.
|
1. DNA (Deoxyribonucleic Acid) (28)
|
|
|
|
One of the basic principles of biology. The main concept of this theory is that traits are passed from parents to offspring through ____ transmission. _____ are located on chromosomes and consist of DNA.
|
1. Gene Theory (28)
|
|
|
|
The genetic information of an organism.
|
1. Genome (28)
|
|
|
|
The genes of an organism are inherited as discrete units.
|
1. Theory of Heredity (30)
|
|
|
|
The genes of Mendel’s theory are physically located on chromosomes and that it is because chromosomes are parceled out in a regular manner during reproduction that Mendel’s regular patterns are seen.
|
1. Chromosomal Theory of Inheritance (30)
|
|
|
|
Advanced by Charles Darwin in 1859, attributes the diversity of the living world to natural selection.
|
1. Theory of Evolution (30)
|
|
|
|
1. Biology and the Living World: Biology – the study of ______ things.
|
Biology and the Living World: Biology – the study of [LIVING] things.
|
|
|
|
1. Biology and the Living World: Living things are _______.
|
Biology and the Living World: Living things are [DIVERSE].
|
|
|
|
1. Biology and the Living World: There are enough ____________ among some living things that they can be grouped into the same _______.
|
Biology and the Living World: There are enough [SIMILARITIES] among some living things that they can be grouped into the same [KINGDOM].
|
|
|
|
1. Biology and the Living World: Members of different ________ are usually very different from each other.
|
Biology and the Living World: Members of different [KINGDOMS] are usually very different from each other.
|
|
|
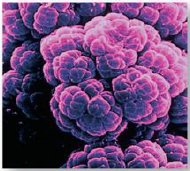
1. The Six Kingdoms of Life: This kingdom of prokaryotes (the simplest of cells that do not have ______) includes this methanogen, which manufactures methane as a result of its metabolic activity. (_______) |
![The Six Kingdoms of Life: This kingdom of prokaryotes (the simplest of cells that do not have [NUCLEI]) includes this methanogen, which manufactures methane as a result of its metabolic activity. ([ARCHAEA])](https://images.cram.com/images/upload-flashcards/51/08/66/6510866_m.png)
The Six Kingdoms of Life: This kingdom of prokaryotes (the simplest of cells that do not have [NUCLEI]) includes this methanogen, which manufactures methane as a result of its metabolic activity. ([ARCHAEA]) |
|
|
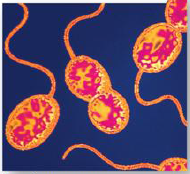
1. The Six Kingdoms of Life: This group is the second of the two prokaryotic kingdoms. Shown here are purple sulfur bacteria, which are able to convert light energy into ________ energy. (________) |
![The Six Kingdoms of Life: This group is the second of the two prokaryotic kingdoms. Shown here are purple sulfur bacteria, which are able to convert light energy into [CHEMICAL] energy. (BACTERIA)](https://images.cram.com/images/upload-flashcards/51/09/23/6510923_m.png)
The Six Kingdoms of Life: This group is the second of the two prokaryotic kingdoms. Shown here are purple sulfur bacteria, which are able to convert light energy into [CHEMICAL] energy. (BACTERIA) |
|
|

1. The Six Kingdoms of Life: Most of the unicellular eukaryotes (those whose cells contain a _______) are grouped into this kingdom, and so are the multicellular algae pictured here. (________) |
![The Six Kingdoms of Life: Most of the unicellular eukaryotes (those whose cells contain a [NUCLEUS]) are grouped into this kingdom, and so are the multicellular algae pictured here. ([PROTISTA])](https://images.cram.com/images/upload-flashcards/51/09/92/6510992_m.png)
The Six Kingdoms of Life: Most of the unicellular eukaryotes (those whose cells contain a [NUCLEUS]) are grouped into this kingdom, and so are the multicellular algae pictured here. ([PROTISTA]) |
|
|

1. The Six Kingdoms of Life: This kingdom contains nonphotosynthetic organisms, mostly multicellular, that digest their food __________, such as these mushrooms. (_____) |
![The Six Kingdoms of Life: This kingdom contains nonphotosynthetic organisms, mostly multicellular, that digest their food [EXTERNALLY], such as these mushrooms. ([FUNGI])](https://images.cram.com/images/upload-flashcards/51/10/46/6511046_m.png)
The Six Kingdoms of Life: This kingdom contains nonphotosynthetic organisms, mostly multicellular, that digest their food [EXTERNALLY], such as these mushrooms. ([FUNGI]) |
|
|

1. The Six Kingdoms of Life: This kingdom contains photosynthetic multicellular organisms that are ___________, such as the flower plant pictured here. (_______) |
![The Six Kingdoms of Life: This kingdom contains photosynthetic multicellular organisms that are [TERRESTRIAL], such as the flower plant pictured here. ([PLANTAE])](https://images.cram.com/images/upload-flashcards/51/10/58/6511058_m.png)
The Six Kingdoms of Life: This kingdom contains photosynthetic multicellular organisms that are [TERRESTRIAL], such as the flower plant pictured here. ([PLANTAE]) |
|
|

1. The Six Kingdoms of Life: Organisms in this kingdom are nonphotosynthetic multicellular organisms that digest their food __________, such as this ram. (________) |
![The Six Kingdoms of Life: Organisms in this kingdom are nonphotosynthetic multicellular organisms that digest their food [INTERNALLY], such as this ram. ([ANIMALIA])](https://images.cram.com/images/upload-flashcards/51/10/67/6511067_m.png)
The Six Kingdoms of Life: Organisms in this kingdom are nonphotosynthetic multicellular organisms that digest their food [INTERNALLY], such as this ram. ([ANIMALIA]) |
|
|
|
1. Cellular organization – composed of ___ or ____ cells.
|
Cellular organization – composed of [ONE] or [MORE] cells. |
|
|
|
1. Metabolism – use of ______ to power processes.
|
Metabolism – use of [ENERGY] to power processes.
|
|
|
|
1. Homeostasis – maintain stable ________ conditions so that their complex _________ can be better coordinated.
|
Homeostasis – maintain stable [INTERNAL] conditions so that their complex [PROCESSES] can be better coordinated.
|
|
|
|
1. Growth and Reproduction – able to ____ and _________.
|
Growth and Reproduction – able to [GROW] and [REPRODUCE].
|
|
|
|
1. Heredity – genetic system that is based on the ___________ and ___________ of DNA. |
Heredity – genetic system that is based on the [REPLICATION] and [DUPLICATION] of DNA. |
|
|
1. Is it alive? Escherichia coli bacteria (e.coli) |
Is it alive? Escherichia coli bacteria (e.coli) |
|
|

1. Is it alive? Rocks? Moss? |

Is it alive? Rocks? Moss? |
|
|

1. Is it alive? Robot? |

Is it alive? Robot? |
|
|

1. Is it alive? Ocean life? |

Is it alive? Ocean life? |
|
|

1. Is it alive? Firn? |

Is it alive? Firn? |
|
|

1. Is it alive? Clam? |

Is it alive? Clam? |
|
|

|
|
|
|

|

|
|
|
|

|
|
|
|
|
|
|
|
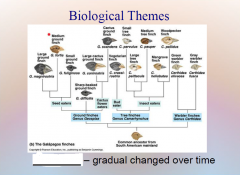
1. Biological Themes: Evolution – _______ change in a species over ____.
|
Biological Themes: Evolution – [GENETIC] change in a species over [TIME].
|
|
|
|
1. Biological Themes: Flow of Energy – from ___ to plants to plant eating _______, etc.
|
Biological Themes: Flow of Energy – from [SUN] to plants to plant eating [ANIMALS], etc.
|
|
|
|
1. Biological Themes: Cooperation – between 2 _________.
|
Biological Themes: Cooperation – between 2 [ORGANISMS].
|
|
|
|
1. Biological Themes: Structure Determines Function – the way something is __________ allows for a specific function(s).
|
Biological Themes: Structure Determines Function – the way something is [STRUCTURED] allows for a specific function(s).
|
|
|
|
1. Biological Themes: Homeostasis – relatively ______ internal environment.
|
Biological Themes: Homeostasis – relatively [STABLE] internal environment.
|
|
|

|

|
|
|

|

|
|
|
|

|
|
|

|

|
|
|

|

|
|
|
|
1. How Scientists Think: Deductive Reasoning – make individual decisions by applying a “_____” of accepted general principles.
|
How Scientists Think: Deductive Reasoning – make individual decisions by applying a “[GUIDE]” of accepted general principles.
|
|
|
|
1. How Scientists Think: Inductive Reasoning – discovering general principles by careful ___________.
|
How Scientists Think: Inductive Reasoning – discovering general principles by careful [EXAMINATION].
|
|
|
|
1. How Scientists Think: What Kind of Reasoning? I left for my first day of class 20 minutes before it started and got there on time. I should leave 20 minutes before class every day to get there on time.
|
Inductive Reasoning, forming a general principle (or hypothesis) that it takes 20 minutes to get to class on time.
|
|
|
|
1. How Scientists Think: What Kind of Reasoning? Every day I leave 20 minutes before class and arrive on time.
|
Deductive Reasoning, based on the general principle that it will take the same amount of time to get to school every day if traffic and road conditions are normal.
|
|
|

1. Science in Action: Scientist Joseph ______ observed in 1985 that ozone levels in Antarctica were alarmingly low (30% drop since 1980) – Pollution by chlorofluorocarbons (CFCs) was later found to be the culprit – CFCs were common components of many _________ products, such as: coolants used in air conditioners, propellants in aerosols, and foaming agents in Styrofoam. |
![Science in Action: Scientist Joseph [FARMAN] observed in 1985 that ozone levels in Antarctica were alarmingly low (30% drop since 1980) – Pollution by chlorofluorocarbons (CFCs) was later found to be the culprit – CFCs were common components o...](https://images.cram.com/images/upload-flashcards/51/14/75/6511475_m.png)
Science in Action: Scientist Joseph [FARMAN] observed in 1985 that ozone levels in Antarctica were alarmingly low (30% drop since 1980) – Pollution by chlorofluorocarbons (CFCs) was later found to be the culprit – CFCs were common components of many [SYNTHETIC] products, such as: coolants used in air conditioners, propellants in aerosols, and foaming agents in Styrofoam. |
|
|
|
1. Science in Action: Ozone depletion is a serious matter: ozone acts as a sunscreen against ___________ rays from the sun, and a 1% drop in ozone leads to a _% increase in skin cancer. A world-wide reduction in CFC production has helped alleviate ozone depletion.
|
Science in Action: Ozone depletion is a serious matter: ozone acts as a sunscreen against [ULTRAVIOLET] rays from the sun, and a 1% drop in ozone leads to a [6]% increase in skin cancer. A world-wide reduction in CFC production has helped alleviate ozone depletion.
|
|
|
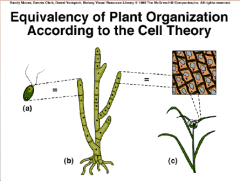
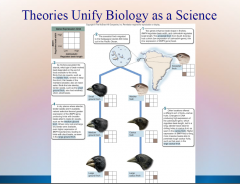
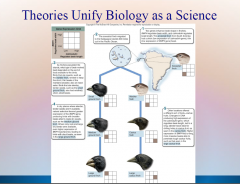
1. Theories Unify Biology as a Science: Cell Theory: Organization of Life – 1839 Matthias _________ and Theodor Schwann concluded that all living organisms consist of _____. |
![Theories Unify Biology as a Science: Cell Theory: Organization of Life – 1839 Matthias [SCHLEIDEN] and Theodor Schwann concluded that all living organisms consist of [CELLS].](https://images.cram.com/images/upload-flashcards/51/16/73/6511673_m.png)
Theories Unify Biology as a Science: Cell Theory: Organization of Life – 1839 Matthias [SCHLEIDEN] and Theodor Schwann concluded that all living organisms consist of [CELLS]. |
|
|
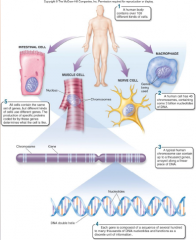
1. Theories Unify Biology as a Science: Gene Theory: Molecular Basis of Inheritance – ________ and RNA molecules are encoded by an organisms’ _____ (portions of DNA) that determine what an organism is like. |
![Theories Unify Biology as a Science: Gene Theory: Molecular Basis of Inheritance – [PROTEINS] and RNA molecules are encoded by an organisms’ [GENES] (portions of DNA) that determine what an organism is like.](https://images.cram.com/images/upload-flashcards/51/16/85/6511685_m.png)
Theories Unify Biology as a Science: Gene Theory: Molecular Basis of Inheritance – [PROTEINS] and RNA molecules are encoded by an organisms’ [GENES] (portions of DNA) that determine what an organism is like. |
|
|
1. Theories Unify Biology as a Science: Theory of Heredity: Unity of Life – 1865 Gregor ______ stated that genes of an organism are _________ as discrete units. |
![Theories Unify Biology as a Science: Theory of Heredity: Unity of Life – 1865 Gregor [MENDEL] stated that genes of an organism are [INHERITED] as discrete units.](https://images.cram.com/images/upload-flashcards/51/18/50/6511850_m.png)
Theories Unify Biology as a Science: Theory of Heredity: Unity of Life – 1865 Gregor [MENDEL] stated that genes of an organism are [INHERITED] as discrete units. |
|
|
|
1. Theories Unify Biology as a Science: Theory of Evolution: Diversity of Life – 1859 Charles ______ attributed the diversity of the living world to _______ _________.
|
Theories Unify Biology as a Science: Theory of Evolution: Diversity of Life – 1859 Charles [DARWIN] attributed the diversity of the living world to [NATURAL SELECTION].
|
|
|
|
1. Theories Unify Biology as a Science: Theory of Evolution: Diversity of Life – Natural Selection – the differential ____________ of genotypes caused by factors in the environment. Leads to ____________ change.
|
Theories Unify Biology as a Science: Theory of Evolution: Diversity of Life – Natural Selection – the differential [REPRODUCTION] of genotypes caused by factors in the environment. Leads to [EVOLUTIONARY] change.
|
|
|
|
|
|
|
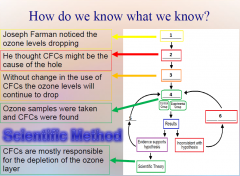
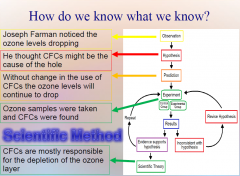
1. How do we know what we know? Scientists try to establish what is true by investigating in ______. Form general __________ through inductive and deductive reasoning. Remember Joseph Farman and CFCs? |
![How do we know what we know? Scientists try to establish what is true by investigating in [STAGES]. Form general [PRINCIPLES] through inductive and deductive reasoning. Remember Joseph Farman and CFCs?](https://images.cram.com/images/upload-flashcards/51/18/83/6511883_m.png)
How do we know what we know? Scientists try to establish what is true by investigating in [STAGES]. Form general [PRINCIPLES] through inductive and deductive reasoning. Remember Joseph Farman and CFCs? |
|
|
|
1. How do we know what we know? Joseph Farman followed a process called the __________ ______. He investigated what he thought was a “true general _________” using the scientific method stages: Observation, Hypothesis, Predictions, Testing, Controls, and Conclusion.
|
How do we know what we know? Joseph Farman followed a process called the [SCIENTIFIC METHOD]. He investigated what he thought was a “true general [PRINCIPLE]” using the scientific method stages: Observation, Hypothesis, Predictions, Testing, Controls, and Conclusion.
|
|
|
|
|
|
|
|
1. Biology is the study of ______ things – the science of life. (16)
|
Biology is the study of [LIVING] things – the science of life. (16)
|
|
|
|
1. The living world is full of a variety of creatures – whales, butterflies, mushrooms, and mosquitoes – all of which can be categorized into six groups, or kingdoms, of organisms: _______, ________, ________, _____, _______, and ________. (16)
|
The living world is full of a variety of creatures – whales, butterflies, mushrooms, and mosquitoes – all of which can be categorized into six groups, or kingdoms, of organisms: [ARCHAEA], [BACTERIA], [PROTISTA], [FUNGI], [PLANTAE], and [ANIMALIA]. (16) |
|
|
|
1. All ______ ______ have much in common. (16)
|
All [LIVING THINGS] have much in common. (16)
|
|
|
|
1. Archaea. This kingdom of prokaryotes (the simplest of cells that do not have ______) includes this methanogen, which manufactures methane as a result of its metabolic activity. (16)
|
Archaea. This kingdom of prokaryotes (the simplest of cells that do not have [NUCLEI]) includes this methanogen, which manufactures methane as a result of its metabolic activity. (16)
|
|
|
|
1. Bacteria. This group is the second of the two ___________ kingdoms. Shown here are purple sulfur bacteria, which are able to convert light energy into chemical energy. (16)
|
Bacteria. This group is the second of the two [PROKARYOTIC] kingdoms. Shown here are purple sulfur bacteria, which are able to convert light energy into chemical energy. (16)
|
|
|
|
1. Protista. Most of the unicellular eukaryotes (those whose cells contain a _______) are grouped into this kingdom, and so are the multicellular algae pictured here. (16)
|
Protista. Most of the unicellular eukaryotes (those whose cells contain a [NUCLEUS]) are grouped into this kingdom, and so are the multicellular algae pictured here. (16)
|
|
|
|
1. Fungi. This kingdom contains _________________ organisms, mostly multicellular, that digest their food externally, such as these mushrooms. (16)
|
Fungi. This kingdom contains [NONPHOTOSYNTHETIC] organisms, mostly multicellular, that digest their food externally, such as these mushrooms. (16)
|
|
|
|
1. Plantae. This kingdom contains photosynthetic _____________ organisms that are terrestrial, such as the flowering plant pictured here. (16)
|
Plantae. This kingdom contains photosynthetic [MULTICELLULAR] organisms that are terrestrial, such as the flowering plant pictured here. (16)
|
|
|
|
1. Animalia. Organisms in this kingdom are _________________ multicellular organisms that digest their food internally, such as this ram. (16)
|
Animalia. Organisms in this kingdom are [NONPHOTOSYNTHETIC] multicellular organisms that digest their food internally, such as this ram. (16)
|
|
|
|
1. Key Learning Outcome 1.1 – The ______ _____ is very diverse, but all things share many key properties. (16)
|
Key Learning Outcome 1.1 – The [LIVING WORLD] is very diverse, but all things share many key properties. (16)
|
|
|
|
1. Figure 1.1 – The six kingdoms of life. Biologists assign all living things to six major categories called ________. Each kingdom is profoundly different from the others. (16)
|
Figure 1.1 – The six kingdoms of life. Biologists assign all living things to six major categories called [KINGDOMS]. Each kingdom is profoundly different from the others. (16)
|
|
|
|
1. All living things share five basic properties, passed down over millions of years from the first organisms to evolve on earth: ________ ____________, __________, ___________, ______ ___ ____________, and ________. (17)
|
All living things share five basic properties, passed down over millions of years from the first organisms to evolve on earth: [CELLULAR ORGANIZATION], [METABOLISM], [HOMEOSTASIS], [GROWTH] and [REPRODUCTION], and [HEREDITY]. (17)
|
|
|
|
1. Cellular Organization (a). All living things are composed of one or more _____. (17)
|
Cellular Organization (a). All living things are composed of one or more [CELLS]. (17)
|
|
|
|
1. Cellular Organization (b). A cell is a tiny compartment with a thin covering called a ________. (17)
|
Cellular Organization (b). A cell is a tiny compartment with a thin covering called a [MEMBRANE]. (17)
|
|
|
|
1. Cellular Organization (c). Some cells have simple _________, while others are complexly organized, but all are able to grow and reproduce. (17)
|
Cellular Organization (c). Some cells have simple [INTERIORS], while others are complexly organized, but all are able to grow and reproduce. (17)
|
|
|
1. Cellular Organization (d). Many _________ possess only a single cell, like the paramecia in figure 1.2; your body contains from 10 trillion to 100 trillion cells (depending on how big you are). (17) |
![Cellular Organization (d). Many [ORGANISMS] possess only a single cell, like the paramecia in figure 1.2; your body contains from 10 trillion to 100 trillion cells (depending on how big you are). (17)](https://images.cram.com/images/upload-flashcards/51/23/09/6512309_m.png)
Cellular Organization (d). Many [ORGANISMS] possess only a single cell, like the paramecia in figure 1.2; your body contains from 10 trillion to 100 trillion cells (depending on how big you are). (17) |
|
|
|
1. Metabolism (a). All living things use energy. Moving, growing, thinking – everything you do requires energy. Where does all this energy come from? (17)
|
It is captured from sunlight by plants and algae through photosynthesis. (17)
|
|
|

1. Metabolism (b). To get the energy that powers our lives we extract it from ______ or from plant-eating _______. That’s what the kingfisher is doing in figure 1.3, eating a fish that ate algae. (17) |
![Metabolism (b). To get the energy that powers our lives we extract it from [PLANTS] or from plant-eating [ANIMALS]. That’s what the kingfisher is doing in figure 1.3, eating a fish that ate algae. (17)](https://images.cram.com/images/upload-flashcards/51/23/66/6512366_m.png)
Metabolism (b). To get the energy that powers our lives we extract it from [PLANTS] or from plant-eating [ANIMALS]. That’s what the kingfisher is doing in figure 1.3, eating a fish that ate algae. (17) |
|
|
|
1. Metabolism (c). The transfer of energy from one form to another in _____ is an example of metabolism. (17)
|
Metabolism (c). The transfer of energy from one form to another in [CELLS] is an example of metabolism. (17)
|
|
|
|
1. Metabolism (d). All organisms require energy to grow, and all organisms transfer this energy from one place to another within cells using special energy-carrying molecules called ___ molecules. (17)
|
Metabolism (d). All organisms require energy to grow, and all organisms transfer this energy from one place to another within cells using special energy-carrying molecules called [ATP] molecules. (17)
|
|
|
|
1. Homeostasis (a). All living things maintain stable ________ conditions so that their complex processes can be better coordinated. (17)
|
Homeostasis (a). All living things maintain stable [INTERNAL] conditions so that their complex processes can be better coordinated. (17)
|
|
|
|
1. Homeostasis (b). While the environment often varies a lot, organisms act to keep their interior conditions relatively constant; a process called ___________. (17)
|
Homeostasis (b). While the environment often varies a lot, organisms act to keep their interior conditions relatively constant; a process called [HOMEOSTASIS]. (17)
|
|
|
|
1. Homeostasis (c). Your body acts to maintain an ________ ___________ of 37˚C (98.6˚F), however hot or cold the weather might be. (17)
|
Homeostasis (c). Your body acts to maintain an [INTERNAL TEMPERATURE] of 37˚C (98.6˚F), however hot or cold the weather might be. (17)
|
|
|
|
1. Growth and reproduction (a). All living things grow and reproduce. ________ increase in size and simply split in two as often as every 15 minutes, while more complex _________ grow by increasing the number of cells and reproduce sexually (some, like the bristlecone pine of California, have reproduced after 4,600 years). (17)
|
Growth and reproduction (a). All living things grow and reproduce. [BACTERIA] increase in size and simply split in two as often as every 15 minutes, while more complex [ORGANISMS] grow by increasing the number of cells and reproduce sexually (some, like the bristlecone pine of California, have reproduced after 4,600 years). (17)
|
|
|
|
1. Heredity. All organisms possess a genetic system that is based on the replication and ___________ of a long molecule called DNA (deoxyribonucleic acid). (17)
|
Heredity. All organisms possess a genetic system that is based on the replication and [DUPLICATION] of a long molecule called DNA (deoxyribonucleic acid). (17)
|
|
|
|
1. The information that determines what an individual organism will be like is contained in a code that is dictated by the order of the subunits making up the ___ molecule, just as the order of letters on this page determines the sense of what you are reading. (17)
|
The information that determines what an individual organism will be like is contained in a code that is dictated by the order of the subunits making up the [DNA] molecule, just as the order of letters on this page determines the sense of what you are reading. (17)
|
|
|
|
1. Each set of instructions within the DNA is called a ____. (17)
|
Each set of instructions within the DNA is called a [GENE]. (17)
|
|
|
|
1. Together, the genes determine what the organism will be like. Because DNA is faithfully copied from one generation to the next, any change in a gene is also preserved and passed on to ______ ___________. (17)
|
Together, the genes determine what the organism will be like. Because DNA is faithfully copied from one generation to the next, any change in a gene is also preserved and passed on to [FUTURE GENERATIONS]. (17)
|
|
|
|
1. The transmission of characteristics from parent to offspring is a process called ________. (17)
|
The transmission of characteristics from parent to offspring is a process called [HEREDITY]. (17)
|
|
|
|
1. Learning Objective 1.2.1 – List the five basic properties shared by all living things: ________ ____________, metabolism, ___________, growth and reproduction, and ________. (17)
|
Learning Objective 1.2.1 – List the five basic properties shared by all living things: [CELLULAR ORGANIZATION], metabolism, [HOMEOSTASIS], growth and reproduction, and [HEREDITY]. (17)
|
|
|
1. Figure 1.2 – Cellular organization. These paramecia are complex single-celled protists that have just ingested several yeast cells. Like these paramecia, many _________ consist of just a single cell, while others are composed of trillions of cells. (17) |
![Figure 1.2 – Cellular organization. These paramecia are complex single-celled protists that have just ingested several yeast cells. Like these paramecia, many [ORGANISMS] consist of just a single cell, while others are composed of trillions of c...](https://images.cram.com/images/upload-flashcards/51/24/05/6512405_m.png)
Figure 1.2 – Cellular organization. These paramecia are complex single-celled protists that have just ingested several yeast cells. Like these paramecia, many [ORGANISMS] consist of just a single cell, while others are composed of trillions of cells. (17) |
|
|

1. Figure 1.3 – Metabolism. This kingfisher obtains the energy it needs to move, grow, and carry out its body processes by eating fish. It metabolizes this food using ________ processes that occur within cells. (17) |
![Figure 1.3 – Metabolism. This kingfisher obtains the energy it needs to move, grow, and carry out its body processes by eating fish. It metabolizes this food using [CHEMICAL] processes that occur within cells. (17)](https://images.cram.com/images/upload-flashcards/51/24/29/6512429_m.png)
Figure 1.3 – Metabolism. This kingfisher obtains the energy it needs to move, grow, and carry out its body processes by eating fish. It metabolizes this food using [CHEMICAL] processes that occur within cells. (17) |
|
|
|
1. Key Learning Outcome 1.2 – All ______ ______ possess cells that carry out metabolism, maintain stable internal conditions, reproduce themselves, and use DNA to transmit hereditary information to offspring. (17)
|
Key Learning Outcome 1.2 – All [LIVING THINGS] possess cells that carry out metabolism, maintain stable internal conditions, reproduce themselves, and use DNA to transmit hereditary information to offspring. (17)
|
|
|
|
1. Learning Objective 1.3.1 – List the 13 hierarchal levels of the organization of life: _____, _________, ______________, __________, _____, _______, ______, _____ _______, ________, __________, _______, _________, and _________. (18)
|
Learning Objective 1.3.1 – List the 13 hierarchal levels of the organization of life: [ATOMS], [MOLECULES], [MACROMOLECULES], [ORGANELLES], [CELLS], [TISSUES], [ORGANS], [ORGAN SYSTEMS], [ORGANISM], [POPULATION], [SPECIES], [COMMUNITY], and [ECOSYSTEM]. (18) |
|
|
|
1. The complexity of life is categorized in three levels: ________, __________, and ____________. (18)
|
The complexity of life is categorized in three levels: [CELLULAR], [ORGANISMAL], and [POPULATIONAL]. (18) |
|
|
|
1. The complexity of life is categorized in three levels: cellular (_____, _________, ______________, __________, and _____). (18)
|
The complexity of life is categorized in three levels: cellular ([ATOMS], [MOLECULES], [MACROMOLECULES], [ORGANELLES], and [CELLS]). (18) |
|
|
|
1. The complexity of life is categorized in three levels: organismal (_______, ______, _____ _______, and ________). (18)
|
The complexity of life is categorized in three levels: organismal ([TISSUES], [ORGANS], [ORGAN SYSTEMS], and [ORGANISM]). (18)
|
|
|
|
1. The complexity of life is categorized in three levels: populational (__________, _______, _________, and _________). (18)
|
The complexity of life is categorized in three levels: populational ([POPULATION], [SPECIES], [COMMUNITY], and [ECOSYSTEM]). (18)
|
|
|

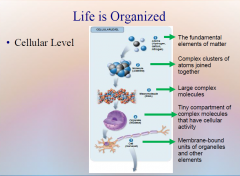
1. Cellular Level. Following down the first section of figure 1.4, you can see that structures get more and more complex – that there is a _________ of increasing complexity within cells. (18) |
![Cellular Level. Following down the first section of figure 1.4, you can see that structures get more and more complex – that there is a [HIERARCHY] of increasing complexity within cells. (18)](https://images.cram.com/images/upload-flashcards/51/24/59/6512459_m.jpg)
Cellular Level. Following down the first section of figure 1.4, you can see that structures get more and more complex – that there is a [HIERARCHY] of increasing complexity within cells. (18) |
|
|
|
1. Cellular Level: Atoms. The fundamental elements of ______ are atoms. (18)
|
Cellular Level: Atoms. The fundamental elements of [MATTER] are atoms. (18)
|
|
|
|
1. Cellular Level: Molecules. Atoms are joined together into complex clusters called _________. (18)
|
Cellular Level: Molecules. Atoms are joined together into complex clusters called [MOLECULES]. (18)
|
|
|
|
1. Cellular Level: Macromolecules. Large _______ molecules are called macromolecules. DNA, which stores the hereditary information in all living _________, is a macromolecule. (18)
|
Cellular Level: Macromolecules. Large [COMPLEX] molecules are called macromolecules. DNA, which stores the hereditary information in all living [ORGANISMS], is a macromolecule. (18)
|
|
|
|
1. Cellular Level: Organelles. Complex biological molecules are assembled into tiny ____________ within cells called organelles, within which cellular activities are organized. (18)
|
Cellular Level: Organelles. Complex biological molecules are assembled into tiny [COMPARTMENTS] within cells called organelles, within which cellular activities are organized. (18)
|
|
|
|
1. The _______ is an organelle within which the cell’s DNA is stored. (18)
|
The [NUCLEUS] is an organelle within which the cell’s DNA is stored. (18)
|
|
|
|
1. Cellular Level: Cells. Organelles and other ________ are assembled in the membrane-bounded units we call cells. Cells are the ________ level of organization that can be considered alive. (18)
|
Cellular Level: Cells. Organelles and other [ELEMENTS] are assembled in the membrane-bounded units we call cells. Cells are the [SMALLEST] level of organization that can be considered alive. (18)
|
|
|
|
1. Organismal Level. At the organismal level, in the second section of figure 1.4, _____ are organized into four levels of complexity. (18)
|
Organismal Level. At the organismal level, in the second section of figure 1.4, [CELLS] are organized into four levels of complexity. (18)
|
|
|
|
1. Organismal Level: Tissues. The most basic level is that of _______, which are groups of similar cells that act as a functional unit. (18)
|
Organismal Level: Tissues. The most basic level is that of [TISSUES], which are groups of similar cells that act as a functional unit. (18)
|
|
|
|
1. Nerve tissue is one kind of tissue, composed of cells called _______ that are specialized to carry electrical signals from one place to another in the body. (19)
|
Nerve tissue is one kind of tissue, composed of cells called [NEURONS] that are specialized to carry electrical signals from one place to another in the body. (19)
|
|
|

1. Figure 1.4 – Levels of organization. A traditional and very useful method of sorting through the many ways in which the organisms of the living world ________ is to arrange them in terms of levels of organization, proceeding from the very small and simple to the very large and complex. Here we examine organization within the cellular, organismal, and populational levels. (18) |
![Figure 1.4 – Levels of organization. A traditional and very useful method of sorting through the many ways in which the organisms of the living world [INTERACT] is to arrange them in terms of levels of organization, proceeding from the very smal...](https://images.cram.com/images/upload-flashcards/51/24/77/6512477_m.jpg)
Figure 1.4 – Levels of organization. A traditional and very useful method of sorting through the many ways in which the organisms of the living world [INTERACT] is to arrange them in terms of levels of organization, proceeding from the very small and simple to the very large and complex. Here we examine organization within the cellular, organismal, and populational levels. (18) |
|
|
|
1. Organismal Level: Organs. Tissues, in turn, are grouped into organs, which are body structures composed of several different tissues grouped together in a __________ and __________ unit. (19)
|
Organismal Level: Organs. Tissues, in turn, are grouped into organs, which are body structures composed of several different tissues grouped together in a [STRUCTURAL] and [FUNCTIONAL] unit. (19)
|
|
|
|
1. Your brain is an organ composed of _____ _____ and a variety of connective tissues that form protective coverings and distribute _____. (19)
|
Your brain is an organ composed of [NERVE CELLS] and a variety of connective tissues that form protective coverings and distribute [BLOOD]. (19)
|
|
|
|
1. Organismal Level: Organ Systems. At the third level of organization, organs are _______ into organ systems. The nervous system, for example, consists of sensory organs, the brain and spinal cord, neurons that convey signals to and from them, and supporting cells. (19)
|
Organismal Level: Organ Systems. At the third level of organization, organs are [GROUPED] into organ systems. The nervous system, for example, consists of sensory organs, the brain and spinal cord, neurons that convey signals to and from them, and supporting cells. (19)
|
|
|
|
1. Organismal Level: Organism. Separate _____ _______ function together to form an organism. (19)
|
Organismal Level: Organism. Separate [ORGAN SYSTEMS] function together to form an organism. (19)
|
|
|
|
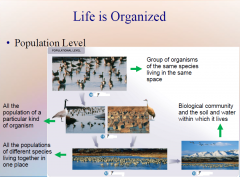
1. Populational Level. Organisms are further organized into several ____________ levels within the living world, as you can see in the third section of figure 1.4. (19)
|
Populational Level. Organisms are further organized into several [HIERARCHICAL] levels within the living world, as you can see in the third section of figure 1.4. (19)
|
|
|
|
1. Populational Level: Population. The most basic of these is the population, which is a group of organisms of the ____ _______ living in the same place. A flock of geese living together on a pond is a population. (19)
|
Populational Level: Population. The most basic of these is the population, which is a group of organisms of the [SAME SPECIES] living in the same place. A flock of geese living together on a pond is a population. (19)
|
|
|
|
1. Populational Level: Species. All the populations of a particular kind of ________ together form a species, its members similar in appearance and able to __________. All Canada geese, whether found in Canada, Minnesota, or Missouri, are basically the same, members of the species Branta Canadensis. Sandhill cranes are a different species. (19)
|
Populational Level: Species. All the populations of a particular kind of [ORGANISM] together form a species, its members similar in appearance and able to [INTERBREED]. All Canada geese, whether found in Canada, Minnesota, or Missouri, are basically the same, members of the species Branta Canadensis. Sandhill cranes are a different species. (19)
|
|
|
|
1. Populational Level: Community. At a higher level of biological organization, a community consists of all the populations of _________ species living ________ in one place. Geese, for example, may share their pond with ducks, fish, grasses, and many kinds of insects. All interact in a single pond community. (19)
|
Populational Level: Community. At a higher level of biological organization, a community consists of all the populations of [DIFFERENT] species living [TOGETHER] in one place. Geese, for example, may share their pond with ducks, fish, grasses, and many kinds of insects. All interact in a single pond community. (19)
|
|
|
|
1. Populational Level: Ecosystem. At the highest tier of biological organization, a biological _________ and the soil and water within which it lives together constitute an ecological system, or ecosystem. (19)
|
Populational Level: Ecosystem. At the highest tier of biological organization, a biological [COMMUNITY] and the soil and water within which it lives together constitute an ecological system, or ecosystem. (19) |
|
|
|
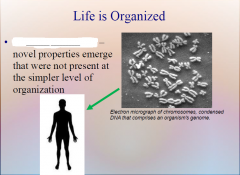
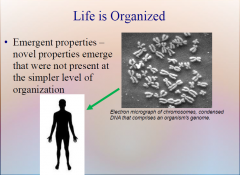
1. At each higher level in the living hierarchy, novel properties emerge that were not present at the simpler level of organization. These ________ __________ result from the way in which components interact and often cannot be guessed just by looking at the parts themselves. (19)
|
At each higher level in the living hierarchy, novel properties emerge that were not present at the simpler level of organization. These [EMERGENT PROPERTIES] result from the way in which components interact and often cannot be guessed just by looking at the parts themselves. (19)
|
|
|
|
1. The emergent properties of life are the natural consequence of the hierarchy, or __________ ____________, that is the hallmark of life. Functional properties emerge from more complex organization. (19)
|
The emergent properties of life are the natural consequence of the hierarchy, or [STRUCTURAL ORGANIZATION], that is the hallmark of life. Functional properties emerge from more complex organization. (19)
|
|
|
|
1. __________ is an emergent property of life. The chemical reactions within a cell arise from interactions between molecules that are orchestrated by the orderly environment of the cell’s ________. (19)
|
[METABOLISM] is an emergent property of life. The chemical reactions within a cell arise from interactions between molecules that are orchestrated by the orderly environment of the cell’s [INTERIOR]. (19)
|
|
|
|
1. Consciousness is an emergent property of the brain that results from the interactions of many _______ in different parts of the brain. (19)
|
Consciousness is an emergent property of the brain that results from the interactions of many [NEURONS] in different parts of the brain. (19)
|
|
|
|
1. Key Learning Outcome 1.3 – Cells, multicellular organisms, and ecological systems each are organized in a hierarchy or increased __________. Life’s hierarchal organization is responsible for the ________ __________ that characterize so many aspects of the living world. (19)
|
Key Learning Outcome 1.3 – Cells, multicellular organisms, and ecological systems each are organized in a hierarchy or increased [COMPLEXITY]. Life’s hierarchal organization is responsible for the [EMERGENT PROPERTIES] that characterize so many aspects of the living world. (19)
|
|
|
|
1. Learning Objective 1.4.1 – List the five general themes that unify biology as a science: _________, ___ ___ __ ______, ___________, _________ __________ ________, and ___________. (20)
|
Learning Objective 1.4.1 – List the five general themes that unify biology as a science: [EVOLUTION], [THE FLOW OF ENERGY], [COOPERATION], [STRUCTURE DETERMINES FUNCTION], and [HOMEOSTASIS]. (20)
|
|
|
|
1. Evolution is _______ change in a species over time. (20)
|
Evolution is [GENETIC] change in a species over time. (20)
|
|
|
|
1. Charles Darwin was an English naturalist who, in 1859, proposed the idea that this change is a result of a process called _______ _________. Simply stated, those organisms whose characteristics make them better able to survive the challenges of their environment live to _________, passing their favorable characteristics on to their offspring. (20)
|
Charles Darwin was an English naturalist who, in 1859, proposed the idea that this change is a result of a process called [NATURAL SELECTION]. Simply stated, those organisms whose characteristics make them better able to survive the challenges of their environment live to [REPRODUCE], passing their favorable characteristics on to their offspring. (20)
|
|
|
|
1. Darwin was thoroughly familiar with variation in domesticated animals (in addition to many nondomesticated organisms), and he knew that varieties of pigeons could be selected by breeders to exhibit exaggerated characteristics, a process called __________ _________. (20)
|
Darwin was thoroughly familiar with variation in domesticated animals (in addition to many nondomesticated organisms), and he knew that varieties of pigeons could be selected by breeders to exhibit exaggerated characteristics, a process called [ARTIFICIAL SELECTION]. (20)
|
|
|
|
1. All organisms require ______ to carry out the activities of living – to build bodies and do work and think thoughts. (20)
|
All organisms require [ENERGY] to carry out the activities of living – to build bodies and do work and think thoughts. (20)
|
|
|
|
1. All of the ______ used by most organisms comes from the sun and is passed in one direction through __________. (20)
|
All of the [ENERGY] used by most organisms comes from the sun and is passed in one direction through [ECOSYSTEMS]. (20)
|
|
|
|
1. The simplest way to understand the ____ of energy trough the living world is to look at who uses it. (20)
|
The simplest way to understand the [FLOW] of energy trough the living world is to look at who uses it. (20)
|
|
|
|
1. The first stage of energy’s journey is its capture by green plants, algae, and some bacteria by the process of ______________. This process uses energy from the sun to synthesize sugars that photosynthetic organisms like plants store in their bodies. Plants then serve as a source of ____-_______ energy for animals that eat them. Other animals, like the eagle in table 1.1, may then eat the plant eaters. (20)
|
The first stage of energy’s journey is its capture by green plants, algae, and some bacteria by the process of [PHOTOSYNTHESIS]. This process uses energy from the sun to synthesize sugars that photosynthetic organisms like plants store in their bodies. Plants then serve as a source of [LIFE-DRIVING] energy for animals that eat them. Other animals, like the eagle in table 1.1, may then eat the plant eaters. (20)
|
|
|
|
1. At each stage, some energy is used for the processes of living, some is transferred, and much is lost, primarily as ____. (20)
|
At each stage, some energy is used for the processes of living, some is transferred, and much is lost, primarily as [HEAT]. (20)
|
|
|
|
1. The flow of energy is a key factor in shaping __________, affecting how many and what kinds of animals live in a community. (20)
|
The flow of energy is a key factor in shaping [ECOSYSTEMS], affecting how many and what kinds of animals live in a community. (20)
|
|
|
|
1. Organisms of two different species that live in direct contact, like the ants and the plant on which they live (table 1.1), form a type of relationship called _________. (20)
|
Organisms of two different species that live in direct contact, like the ants and the plant on which they live (table 1.1), form a type of relationship called [SYMBIOSIS]. (20)
|
|
|
|
1. ______ _____ possess organelles that are the descendants of symbiotic bacteria, and symbiotic fungi helped plants first invade land from the sea. (20)
|
[ANIMAL CELLS] possess organelles that are the descendants of symbiotic bacteria, and symbiotic fungi helped plants first invade land from the sea. (20)
|
|
|
|
1. The coevolution of flowering plants and insects – where changes in flowers influenced insect evolution and, in turn, changes in insects influenced flower evolution – has been responsible for much of life’s great _________. (20)
|
The coevolution of flowering plants and insects – where changes in flowers influenced insect evolution and, in turn, changes in insects influenced flower evolution – has been responsible for much of life’s great [DIVERSITY]. (20)
|
|
|
|
1. One of the most obvious lessons of biology is that biological structures are very well suited to their functions. You will see this at every level of organization: Within cells, the shapes of the proteins called _______ that cells use to carry out ________ reactions are precisely suited to match the chemicals the enzymes must manipulate. (20)
|
One of the most obvious lessons of biology is that biological structures are very well suited to their functions. You will see this at every level of organization: Within cells, the shapes of the proteins called [ENZYMES] that cells use to carry out [CHEMICAL] reactions are precisely suited to match the chemicals the enzymes must manipulate. (20)
|
|
|

1. Within the many kinds of organisms in the living world, body structures seem carefully ________ to carry out their functions – the long tongue with which the moth in table 1.1 sucks nectar from deep within a flower is one example. (20) |
![Within the many kinds of organisms in the living world, body structures seem carefully [DESIGNED] to carry out their functions – the long tongue with which the moth in table 1.1 sucks nectar from deep within a flower is one example. (20)](https://images.cram.com/images/upload-flashcards/51/26/39/6512639_m.jpg)
Within the many kinds of organisms in the living world, body structures seem carefully [DESIGNED] to carry out their functions – the long tongue with which the moth in table 1.1 sucks nectar from deep within a flower is one example. (20) |
|
|
|
1. The superb fit of structure to function in the living world is no accident. Life has existed on earth for over 2 billion years, a long time for _________ to favor changes that better suit organisms to meet the challenges of living. (20)
|
The superb fit of structure to function in the living world is no accident. Life has existed on earth for over 2 billion years, a long time for [EVOLUTION] to favor changes that better suit organisms to meet the challenges of living. (20)
|
|
|
|
1. The high degree of specialization we see among complex organisms is only possible because these organisms act to maintain a relatively ______ internal environment, a process called homeostasis. (20)
|
The high degree of specialization we see among complex organisms is only possible because these organisms act to maintain a relatively [STABLE] internal environment, a process called homeostasis. (20)
|
|
|
|
1. Maintaining homeostasis in a body as complex as your or the hippo’s in table 1.1 requires a great deal of _________ back and forth between cells. (20)
|
Maintaining homeostasis in a body as complex as your or the hippo’s in table 1.1 requires a great deal of [SIGNALING] back and forth between cells. (20)
|
|
|
|
1. Key Learning Outcome 1.4 – The five general themes of biology are (1) evolution, (2) ___ ____ __ ______, (3) cooperation, (4) _________ __________ ________, and (5) homeostasis. (20)
|
Key Learning Outcome 1.4 – The five general themes of biology are (1) evolution, (2) [THE FLOW OF ENERGY], (3) cooperation, (4) [STRUCTURE DETERMINES FUNCTION], and (5) homeostasis. (20)
|
|
|
|
1. Learning Objective 1.5.1 – Identify the form of reasoning used in mathematics and computer science: _________ _________. (21)
|
Learning Objective 1.5.1 – Identify the form of reasoning used in mathematics and computer science: [DEDUCTIVE REASONING]. (21)
|
|
|
|
1. Science is a process of _____________, using observation, experimentation, and reasoning. (21)
|
Science is a process of [INVESTIGATION], using observation, experimentation, and reasoning. (21)
|
|
|
|
1. Not all investigations are scientific. For example, when you want to know how to get to Chicago from St. Louis, you do not conduct a scientific investigation – instead, you look at a map to determine a route. In other investigations, you make individual decisions by applying a “guide” of accepted general principles (figure 1.5). This is called _________ _________. (21)
|
Not all investigations are scientific. For example, when you want to know how to get to Chicago from St. Louis, you do not conduct a scientific investigation – instead, you look at a map to determine a route. In other investigations, you make individual decisions by applying a “guide” of accepted general principles (figure 1.5). This is called [DEDUCTIVE REASONING]. (21)
|
|
|
|
1. _________ _________, using general principles to explain specific observations, is the reasoning of mathematics, philosophy, politics, and ethics; deductive reasoning is also the way a computer works. (21)
|
[DEDUCTIVE REASONING], using general principles to explain specific observations, is the reasoning of mathematics, philosophy, politics, and ethics; deductive reasoning is also the way a computer works. (21)
|
|
|
|
1. Some general principles are derived from observation of the physical world around us. Science is devoted to discovering the general principles that govern the _________ of the physical world. How do scientists discover such general principles: Scientists are, above all, observers: They look at the world to understand how it _____. (21)
|
Some general principles are derived from observation of the physical world around us. Science is devoted to discovering the general principles that govern the [OPERATION] of the physical world. How do scientists discover such general principles: Scientists are, above all, observers: They look at the world to understand how it [WORKS]. (21)
|
|
|
|
1. It is from observations that scientists determine the principles that govern our physical world. This way of discovering general principles by careful examination of specific cases is called _________ _________. (21)
|
It is from observations that scientists determine the principles that govern our physical world. This way of discovering general principles by careful examination of specific cases is called [INDUCTIVE REASONING]. (21)
|
|
|
|
1. Inductive reasoning first became popular about 400 years ago, when Isaac ______, Francis _____, and others began to conduct experiments and from the results infer general principles about how the world ________. (21)
|
Inductive reasoning first became popular about 400 years ago, when Isaac [NEWTON], Francis [BACON], and others began to conduct experiments and from the results infer general principles about how the world [OPERATES]. (21)
|
|
|
|
1. Key Learning Outcome 1.5 – Science uses inductive reasoning to infer general principles from detailed ___________. (21)
|
Key Learning Outcome 1.5 – Science uses inductive reasoning to infer general principles from detailed [OBSERVATION]. (21)
|
|
|
|
1. _________ _________ – An Accepted General Principle – When traffic lights along city streets are “timed” to change at the time interval it takes traffic to pass between them, the result will be a smooth flow of traffic. Traveling at the speed limit, you approach each intersection anticipating that the red light will turn green as you reach the intersection. (21)
|
[DEDUCTIVE REASONING] – An Accepted General Principle – When traffic lights along city streets are “timed” to change at the time interval it takes traffic to pass between them, the result will be a smooth flow of traffic. Traveling at the speed limit, you approach each intersection anticipating that the red light will turn green as you reach the intersection. (21)
|
|
|
|
1. _________ _________ – Observations of Specific Events – Driving down the street at the speed limit, you observe that the red traffic light turns green just as you approach the intersection. Maintaining the same speed, you observe the same event at the next several intersections: the traffic lights turn green just as you approach the intersections. When you speed up, however, the light doesn’t change until after you reach the intersection. Formation of a General Principle – You conclude that the traffic lights along this street are “timed” to change in the time it takes your car, traveling at the speed limit, to traverse the distance between them. (21)
|
[INDUCTIVE REASONING] – Observations of Specific Events – Driving down the street at the speed limit, you observe that the red traffic light turns green just as you approach the intersection. Maintaining the same speed, you observe the same event at the next several intersections: the traffic lights turn green just as you approach the intersections. When you speed up, however, the light doesn’t change until after you reach the intersection. Formation of a General Principle – You conclude that the traffic lights along this street are “timed” to change in the time it takes your car, traveling at the speed limit, to traverse the distance between them. (21)
|
|
|

1. Figure 1.5 – Deductive and Inductive Reasoning – A driver who assumes that the traffic signals are timed can use _________ reasoning to expect that the traffic lights will change predictably at intersections. In contrast, a driver who is not aware of the general control and programming of traffic signals can use _________ reasoning to determine that the traffic lights are timed as the driver encounters similar timing of signals at several intersections. (21) |
![Figure 1.5 – Deductive and Inductive Reasoning – A driver who assumes that the traffic signals are timed can use [DEDUCTIVE] reasoning to expect that the traffic lights will change predictably at intersections. In contrast, a driver who is not...](https://images.cram.com/images/upload-flashcards/51/26/66/6512666_m.jpg)
Figure 1.5 – Deductive and Inductive Reasoning – A driver who assumes that the traffic signals are timed can use [DEDUCTIVE] reasoning to expect that the traffic lights will change predictably at intersections. In contrast, a driver who is not aware of the general control and programming of traffic signals can use [INDUCTIVE] reasoning to determine that the traffic lights are timed as the driver encounters similar timing of signals at several intersections. (21) |
|
|
|
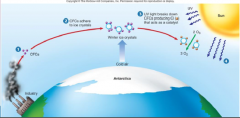
1. Figure 1.6 – How CFCs attack and destroy ozone – CFCs are stable chemicals that accumulate in the atmosphere as a by-product of industrial society (1). In the intense cold of the Antarctic, these CFCs adhere to tiny ice crystals in the upper atmosphere (2). UV light causes the breakdown of CFCs, producing chlorine (Cl). CL acts as a catalyst, converting O3 into O2 (3). As a result, more harmful __ _________ reaches the earth’s surface (4). (23)
|
Figure 1.6 – How CFCs attack and destroy ozone – CFCs are stable chemicals that accumulate in the atmosphere as a by-product of industrial society (1). In the intense cold of the Antarctic, these CFCs adhere to tiny ice crystals in the upper atmosphere (2). UV light causes the breakdown of CFCs, producing chlorine (Cl). CL acts as a catalyst, converting O3 into O2 (3). As a result, more harmful [UV RADIATION] reaches the earth’s surface (4). (23) |
|
|
|
1. In 1985, Joseph ______, a British earth scientist working in Antarctica, discovered by analyzing the Antarctic sky, he found far less ozone (O3, a form of oxygen gas) than should be there – a __% drop from a reading recorded five years earlier in the Antarctic! Evidence soon mounted implicating synthetic chemicals as the culprit. (23)
|
In 1985, Joseph [FARMAN], a British earth scientist working in Antarctica, discovered by analyzing the Antarctic sky, he found far less ozone (O3, a form of oxygen gas) than should be there – a [30]% drop from a reading recorded five years earlier in the Antarctic! Evidence soon mounted implicating synthetic chemicals as the culprit. (23)
|
|
|
|
1. Detailed analysis of chemicals in the Antarctic atmosphere revealed a surprisingly high concentration of chlorine, a chemical known to destroy ozone. The source of the chlorine was a class of chemicals called ___________________ (____). (23)
|
Detailed analysis of chemicals in the Antarctic atmosphere revealed a surprisingly high concentration of chlorine, a chemical known to destroy ozone. The source of the chlorine was a class of chemicals called [CHLOROFLUOROCARBONS (CFCs)]. (23) |
|
|
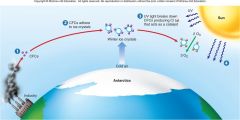
1. CFCs (the purple balls (1) in figure 1.6) have been manufactured in _____ _______ since they were invented in the 1920s, largely for use as coolants in air conditioners, propellants in aerosols, and foaming agents in making Styrofoam. (23) |
![CFCs (the purple balls (1) in figure 1.6) have been manufactured in [LARGE AMOUNTS] since they were invented in the 1920s, largely for use as coolants in air conditioners, propellants in aerosols, and foaming agents in making Styrofoam. (23)](https://images.cram.com/images/upload-flashcards/51/26/75/6512675_m.jpg)
CFCs (the purple balls (1) in figure 1.6) have been manufactured in [LARGE AMOUNTS] since they were invented in the 1920s, largely for use as coolants in air conditioners, propellants in aerosols, and foaming agents in making Styrofoam. (23) |
|
|
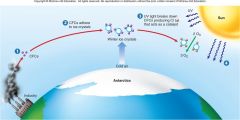
1. CFCs were widely regarded as harmless because they are chemically unreactive under normal conditions. But in the atmosphere over Antarctica, CFCs condense onto tiny ___ ________ (2); in the spring, the CFCs break down and produce chlorine, which acts as a catalyst, attacking and destroying ozone, turning it into ______ ___ without the chlorine being used up (3). (23) |
![CFCs were widely regarded as harmless because they are chemically unreactive under normal conditions. But in the atmosphere over Antarctica, CFCs condense onto tiny [ICE CRYSTALS] (2); in the spring, the CFCs break down and produce chlorine, which...](https://images.cram.com/images/upload-flashcards/51/26/81/6512681_m.jpg)
CFCs were widely regarded as harmless because they are chemically unreactive under normal conditions. But in the atmosphere over Antarctica, CFCs condense onto tiny [ICE CRYSTALS] (2); in the spring, the CFCs break down and produce chlorine, which acts as a catalyst, attacking and destroying ozone, turning it into [OXYGEN GAS] without the chlorine being used up (3). (23) |
|
|
|
1. The thinning of the ozone layer in the upper atmosphere 25 to 40 kilometers above the surface of the earth is a serious matter. The ozone layer protects life from the harmful ___________ (UV) rays from the sun that bombard the earth continuously. (23)
|
The thinning of the ozone layer in the upper atmosphere 25 to 40 kilometers above the surface of the earth is a serious matter. The ozone layer protects life from the harmful [ULTRAVIOLET (UV)] rays from the sun that bombard the earth continuously. (23)
|
|
|
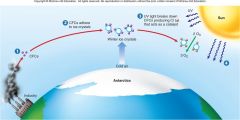
1. Like invisible sunglasses, the ozone layer filters out these dangerous rays. So when ozone is converted to oxygen gas, the UV rays are able to pass through to the earth (4). When UV rays damage the DNA in skin cells, it can lead to skin cancer. It is estimated that every _% drop in the atmospheric ozone concentration leads to a _% increase in skin cancers. (23) |
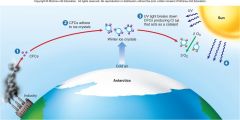
Like invisible sunglasses, the ozone layer filters out these dangerous rays. So when ozone is converted to oxygen gas, the UV rays are able to pass through to the earth (4). When UV rays damage the DNA in skin cells, it can lead to skin cancer. It is estimated that every [1%] drop in the atmospheric ozone concentration leads to a [6%] increase in skin cancers. (23) |
|
|
|
1. The world currently produces fewer than 200,000 tons of CFCs annually, down from ____ levels of _._ million tons. (23)
|
The world currently produces fewer than 200,000 tons of CFCs annually, down from [1986] levels of [1.1] million tons. (23)
|
|
|
|
1. Most of the CFCs manufactured since they were invented are still in use in air conditioners and aerosols and have not yet _______ the atmosphere. As these CFCs move slowly upward through the atmosphere, the problem can be expected to continue. Ozone depletion is _____ producing major ozone holes over the Antarctic. (23)
|
Most of the CFCs manufactured since they were invented are still in use in air conditioners and aerosols and have not yet [REACHED] the atmosphere. As these CFCs move slowly upward through the atmosphere, the problem can be expected to continue. Ozone depletion is [STILL] producing major ozone holes over the Antarctic. (23)
|
|
|
|
1. The worldwide reduction in CFC production is having a _____ impact. The period of maximum ozone depletion will peak in the next few years, and researchers’’ models predict that after that the situation should gradually improve and that the ozone layer will recover by the middle of the 21st century. Clearly, global environmental problems ___ be solved by concerted action. (23)
|
The worldwide reduction in CFC production is having a [MAJOR] impact. The period of maximum ozone depletion will peak in the next few years, and researchers’’ models predict that after that the situation should gradually improve and that the ozone layer will recover by the middle of the 21st century. Clearly, global environmental problems [CAN] be solved by concerted action. (23)
|
|
|
|
1. Key Learning Outcome 1.6 – Industrially produced CFCs catalytically _______ ozone in the upper atmosphere. (23)
|
Key Learning Outcome 1.6 – Industrially produced CFCs catalytically [DESTROY] ozone in the upper atmosphere. (23)
|
|
|
|
1. Scientists establish which general principles are true from among the many that might be by systematically testing ___________ proposals. If these proposals prove inconsistent with experimental observations, they are rejected as untrue. (24)
|
Scientists establish which general principles are true from among the many that might be by systematically testing [ALTERNATIVE] proposals. If these proposals prove inconsistent with experimental observations, they are rejected as untrue. (24)
|
|
|
|
1. After making careful observations concerning a particular area of science, scientists formulate an ___________ that might account for those observations. (24)
|
After making careful observations concerning a particular area of science, scientists formulate an [EXPLANATION] that might account for those observations. (24)
|
|
|
|
1. A hypothesis is a proposition that might be ____. (24)
|
A hypothesis is a proposition that might be [TRUE]. (24)
|
|
|
|
1. Those hypotheses that have not yet been _________ are retained, but they are always subject to future rejection if – in the light of new information – they are found to be _________. (24)
|
Those hypotheses that have not yet been [DISPROVED] are retained, but they are always subject to future rejection if – in the light of new information – they are found to be [INCORRECT]. (24)
|
|
|
|
1. We call the test of a hypothesis an __________. (24)
|
We call the test of a hypothesis an [EXPERIMENT]. (24)
|
|
|
|
1. A successful experiment is one in which one or more hypotheses are demonstrated to be ____________ with the results and are thus rejected. (24)
|
A successful experiment is one in which one or more hypotheses are demonstrated to be [INCONSISTENT] with the results and are thus rejected. (24)
|
|
|
|
1. Biology, like all science, is in a constant state of ______, with new ideas appearing and replacing old ones. (24)
|
Biology, like all science, is in a constant state of [CHANGE], with new ideas appearing and replacing old ones. (24)
|
|
|
|
1. Joseph Farman, who first reported the ozone hole, is a practicing scientist, and what he was doing in Antarctica was science. Science is a particular way of _____________ the world, of forming general rules about why things happen by observing particular situations. (24)
|
Joseph Farman, who first reported the ozone hole, is a practicing scientist, and what he was doing in Antarctica was science. Science is a particular way of [INVESTIGATING] the world, of forming general rules about why things happen by observing particular situations. (24)
|
|
|
|
1. A scientist like Farman is an observer, someone who looks at the world in order to __________ how it works. (24)
|
A scientist like Farman is an observer, someone who looks at the world in order to [UNDERSTAND] how it works. (24)
|
|
|
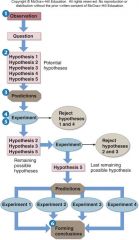
1. Scientific investigations can be said to have six stages as illustrated in figure 1.7: (1) _________ what is going on; (2) forming a set of __________; (3) making ___________; (4) _______ them and (5) carrying out controls, until one or more of the hypotheses have been __________; and (6) forming ___________ based on the remaining hypothesis. (24) |
![Scientific investigations can be said to have six stages as illustrated in figure 1.7: (1) [OBSERVING] what is going on; (2) forming a set of [HYPOTHESES]; (3) making [PREDICTIONS]; (4) [TESTING] them and (5) carrying out controls, until one or mo...](https://images.cram.com/images/upload-flashcards/51/27/92/6512792_m.jpg)
Scientific investigations can be said to have six stages as illustrated in figure 1.7: (1) [OBSERVING] what is going on; (2) forming a set of [HYPOTHESES]; (3) making [PREDICTIONS]; (4) [TESTING] them and (5) carrying out controls, until one or more of the hypotheses have been [ELIMINATED]; and (6) forming [CONCLUSIONS] based on the remaining hypothesis. (24) |
|
|

1. (1) Observation. The key to any successful scientific investigation is careful observation. Farman and other scientists had studied the skies over the Antarctic for many years, noting a thousand details about temperature, light, and levels of chemicals. You can see an example in figure 1.8, where the purple colors represent the lowest levels of ozone that the scientists recorded. Had these scientists not kept careful records of what they ________, Farman might not have noticed that ozone levels were ________. (24) |

(1) Observation. The key to any successful scientific investigation is careful observation. Farman and other scientists had studied the skies over the Antarctic for many years, noting a thousand details about temperature, light, and levels of chemicals. You can see an example in figure 1.8, where the purple colors represent the lowest levels of ozone that the scientists recorded. Had these scientists not kept careful records of what they [OBSERVED], Farman might not have noticed that ozone levels were [DROPPING]. (24) |
|
|
|
1. (2) Hypothesis. When the unexpected drop in ozone was reported and questioned, environmental scientists made a guess to answer their questions – perhaps something was destroying the ozone; maybe the culprit was CFCs. Of course, this was not a guess in the true sense; scientists has some working knowledge of CFCs and what they might be doing in the upper atmosphere. We call such a guess a hypothesis. A hypothesis is a guess that might be ____. (25)
|
(2) Hypothesis. When the unexpected drop in ozone was reported and questioned, environmental scientists made a guess to answer their questions – perhaps something was destroying the ozone; maybe the culprit was CFCs. Of course, this was not a guess in the true sense; scientists has some working knowledge of CFCs and what they might be doing in the upper atmosphere. We call such a guess a hypothesis. A hypothesis is a guess that might be [TRUE].(25)
|
|
|
|
1. What the scientists guessed was that chlorine from CFCs was reacting chemically with ozone over the Antarctic, converting ozone (O3) into oxygen gas (O2) and in the process removing the ozone shield from our earth’s atmosphere. Often, scientists will form ___________ hypotheses if they have more than one guess about what they observe. In this case, there were several other hypotheses advanced to explain the ozone hole. One suggestion explained it as the result of convection: the ozone spun away from the polar regions much as water spins away from the center as a clothes washer moves through its spin cycle. Another hypothesis was that the ozone hole was a transient phenomenon, due perhaps to sunspots, and would soon disappear. (25)
|
What the scientists guessed was that chlorine from CFCs was reacting chemically with ozone over the Antarctic, converting ozone (O3) into oxygen gas (O2) and in the process removing the ozone shield from our earth’s atmosphere. Often, scientists will form [ALTERNATIVE] hypotheses if they have more than one guess about what they observe. In this case, there were several other hypotheses advanced to explain the ozone hole. One suggestion explained it as the result of convection: the ozone spun away from the polar regions much as water spins away from the center as a clothes washer moves through its spin cycle. Another hypothesis was that the ozone hole was a transient phenomenon, due perhaps to sunspots, and would soon disappear. (25)
|
|
|
|
1. (3) Predictions. If the CFC hypothesis is correct, then several consequences can reasonably be expected. We call these expected consequences predictions. A prediction is what you ______ to happen if a hypothesis is true. (25)
|
(3) Predictions. If the CFC hypothesis is correct, then several consequences can reasonably be expected. We call these expected consequences predictions. A prediction is what you [EXPECT] to happen if a hypothesis is true. (25)
|
|
|
|
1. The CFC hypothesis predicts that if CFCs are responsible for producing the ozone hole, then it should be possible to detect CFCs in the upper Antarctic atmosphere as well as the ________ released from CFCs that attach the ozone. (25)
|
The CFC hypothesis predicts that if CFCs are responsible for producing the ozone hole, then it should be possible to detect CFCs in the upper Antarctic atmosphere as well as the [CHLORINE] released from CFCs that attach the ozone. (25)
|
|
|
|
1. (4) Testing. Scientists set out to test the CFC hypothesis by attempting to ______ some of its predictions. As already mentioned, the test of a hypothesis is an experiment. To test the hypothesis, atmospheric samples were collected from the stratosphere over 6 miles up by a high-altitude balloon. Analysis of the samples revealed CFCs, as predicted. Were the CFCs interacting with the ozone? The samples contained free chlorine and fluorine, confirming the breakdown of CFC molecules. The results of the __________ thus support the hypothesis. (25)
|
(4) Testing. Scientists set out to test the CFC hypothesis by attempting to [VERIFY] some of its predictions. As already mentioned, the test of a hypothesis is an experiment. To test the hypothesis, atmospheric samples were collected from the stratosphere over 6 miles up by a high-altitude balloon. Analysis of the samples revealed CFCs, as predicted. Were the CFCs interacting with the ozone? The samples contained free chlorine and fluorine, confirming the breakdown of CFC molecules. The results of the [EXPERIMENT] thus support the hypothesis. (25) |
|
|
|
1. (5) Controls. Events in the upper atmosphere can be influenced by many factors. We call each factor that might influence a process a variable. To evaluate alternative hypotheses about one variable, all the other variables must be kept constant so that we do not get misled or confused by these other influences. This is done by carrying out two experiments in parallel: In the first ____________ test, we alter one variable in a known way to test a particular hypothesis; in the second, called a _______ experiment, we do not alter that variable. In all other respects, the two experiments are the same. (25)
|
(5) Controls. Events in the upper atmosphere can be influenced by many factors. We call each factor that might influence a process a variable. To evaluate alternative hypotheses about one variable, all the other variables must be kept constant so that we do not get misled or confused by these other influences. This is done by carrying out two experiments in parallel: In the first [EXPERIMENTAL] test, we alter one variable in a known way to test a particular hypothesis; in the second, called a [CONTROL] experiment, we do not alter that variable. In all other respects, the two experiments are the same. (25) |
|
|
|
1. To further test the CFC hypothesis, scientists carried out control experiments in which the key ________ was the amount of CFCs in the atmosphere. Working in laboratories, scientists reconstructed the atmospheric conditions, solar bombardment, and extreme temperatures found in the sky far above the Antarctic. If the ozone levels fell without addition of CFCs to the chamber, then CFCs could not be what was attacking the ozone. Carefully monitoring the chamber, however, scientists detected no drop in ozone levels in the _______ of CFCs. (25)
|
To further test the CFC hypothesis, scientists carried out control experiments in which the key [VARIABLE] was the amount of CFCs in the atmosphere. Working in laboratories, scientists reconstructed the atmospheric conditions, solar bombardment, and extreme temperatures found in the sky far above the Antarctic. If the ozone levels fell without addition of CFCs to the chamber, then CFCs could not be what was attacking the ozone. Carefully monitoring the chamber, however, scientists detected no drop in ozone levels in the [ABSENCE] of CFCs. (25) |
|
|
|
1. (6) Conclusion. A hypothesis that has been tested and not ________ is tentatively accepted. The hypothesis that CFCs released into the atmosphere are destroying the earth’s protective ozone shield is now supported by a great deal of ____________ evidence and is widely accepted. While other factors have also been implicated in ozone depletion, destruction by CFCs is clearly the dominant phenomenon. (25)
|
(6) Conclusion. A hypothesis that has been tested and not [REJECTED] is tentatively accepted. The hypothesis that CFCs released into the atmosphere are destroying the earth’s protective ozone shield is now supported by a great deal of [EXPERIMENTAL] evidence and is widely accepted. While other factors have also been implicated in ozone depletion, destruction by CFCs is clearly the dominant phenomenon. (25)
|
|
|
|
1. A collection of related hypotheses that have been tested many times and not rejected is called a ______. A theory indicates a higher degree of certainty; however, in science, nothing is “certain.” The theory of the ozone shield – that ozone in the upper atmosphere shields the earth’s surface from harmful UV rays by absorbing them – is supported by a wealth of ___________ and experimentation and is widely accepted. The explanation for the destruction of this shield is still at the hypothesis stage. (25)
|
A collection of related hypotheses that have been tested many times and not rejected is called a theory. A theory indicates a higher degree of certainty; however, in science, nothing is “certain.” The theory of the ozone shield – that ozone in the upper atmosphere shields the earth’s surface from harmful UV rays by absorbing them – is supported by a wealth of [OBSERVATION] and experimentation and is widely accepted. The explanation for the destruction of this shield is still at the hypothesis stage. (25)
|
|
|
|
1. Key Learning Outcome 1.7 – Science progresses by systematically ___________ potential hypotheses that are not consistent with observation. (25)
|
Key Learning Outcome 1.7 – Science progresses by systematically [ELIMINATING] potential hypotheses that are not consistent with observation. (25)
|
|
|
|
1. A theory is a unifying ___________ for a broad range of observations. Theories are the solid ground of science, that of which we are the most certain. (26)
|
A theory is a unifying [EXPLANATION] for a broad range of observations. Theories are the solid ground of science, that of which we are the most certain. (26)
|
|
|
|
1. There is no absolute truth in science, however, only varying degrees of ___________. The possibility always remains that future evidence will cause a theory to be revised. (26)
|
There is no absolute truth in science, however, only varying degrees of [UNCERTAINTY]. The possibility always remains that future evidence will cause a theory to be revised. (26)
|
|
|
|
1. A scientist’s acceptance of a theory is always ___________. As information is shared throughout the scientific community, previous hypotheses and theories may be modified, and scientists may formulate new ideas. (26)
|
A scientist’s acceptance of a theory is always [PROVISIONAL]. As information is shared throughout the scientific community, previous hypotheses and theories may be modified, and scientists may formulate new ideas. (26)
|
|
|
|
1. Very active areas of science are often alive with ___________, as scientists grope with new and challenging ideas. This uncertainty is not a sign of poor science bur rather of the push and pull that is the heart of the scientific process. (26)
|
Very active areas of science are often alive with [CONTROVERSY], as scientists grope with new and challenging ideas. This uncertainty is not a sign of poor science bur rather of the push and pull that is the heart of the scientific process. (26)
|
|
|
|
1. The word theory is thus used very differently by scientists than by the general public. To a scientist, a theory represents that for which he or she is most _______; to the general public, the word theory implies a lack of knowledge or a guess. How often have you heard someone say, “It’s only a theory!”? (26)
|
The word theory is thus used very differently by scientists than by the general public. To a scientist, a theory represents that for which he or she is most [CERTAIN]; to the general public, the word theory implies a lack of knowledge or a guess. How often have you heard someone say, “It’s only a theory!”? (26)
|
|
|
|
1. It was once fashionable to claim that scientific progress is the result of applying a series of steps called the scientific method; that is, a series of logical “either/or” predictions tested by experiments to reject one alternative. This is ___ true. (26)
|
It was once fashionable to claim that scientific progress is the result of applying a series of steps called the scientific method; that is, a series of logical “either/or” predictions tested by experiments to reject one alternative. This is [NOT] true. (26)
|
|
|
|
1. A hypothesis that a successful scientist tests is not just any hypothesis. Rather, it is a “hunch” or ________ _____ in which the scientist integrates all that he or she knows. It is _______ and imagination that play a large role in scientific progress. (26)
|
A hypothesis that a successful scientist tests is not just any hypothesis. Rather, it is a “hunch” or [EDUCATED GUESS] in which the scientist integrates all that he or she knows. It is [INSIGHT] and imagination that play a large role in scientific progress. (26)
|
|
|
|
1. Scientific study is limited to organisms and _________ that we are able to observe and measure. Supernatural and _________ phenomena are beyond the realm of scientific analysis because they cannot be scientifically studied, analyzed, or explained. (26)
|
Scientific study is limited to organisms and [PROCESSES] that we are able to observe and measure. Supernatural and [RELIGIOUS] phenomena are beyond the realm of scientific analysis because they cannot be scientifically studied, analyzed, or explained. (26)
|
|
|
|
1. While scientific study has revolutionized our world, it cannot be relied upon to _____ all problems. Science identifies solutions to problems when solutions exist, but it cannot ______ solutions when they don’t. (26)
|
While scientific study has revolutionized our world, it cannot be relied upon to [SOLVE] all problems. Science identifies solutions to problems when solutions exist, but it cannot [INVENT] solutions when they don’t. (26)
|
|
|
|
1. Key Learning Outcome 1.8 – A scientist does not follow a fixed method to form hypotheses but relies also on ________ and _________. (26)
|
Key Learning Outcome 1.8 – A scientist does not follow a fixed method to form hypotheses but relies also on [JUDGMENT] and [INTUITION]. (26)
|
|
|
|
1. All organisms are composed of _____, life’s basic units. (28)
|
All organisms are composed of [CELLS], life’s basic units. (28)
|
|
|
|
1. Cells were discovered by Robert _____ in England in 1665, while he was using one of the first microscopes, one that magnified 30 times. Looking through a thin slice of cork, he observed many tiny chambers that reminded him of monks’ cells in a monastery. Not long after that, the Dutch scientist Anton van ___________ used microscopes capable of magnifying 300 times and discovered an amazing world of single-celled life in a drop of pond water like in figure 1.10. He called the bacterial and protist cells he say “wee animalcules.” In 1839, the German biologists Matthias _________ and Theodor _______, summarizing a large number of observations by themselves and others, concluded that all living organisms consist of cells. Their conclusion forms the basis of what has come to be known as the cell theory. Later, biologists added the idea that all cells come from other cells, the cell theory, one of the basic ideas in biology, is the foundation for understanding the reproduction and growth of all organisms. (28)
|
Cells were discovered by Robert [HOOKE] in England in 1665, while he was using one of the first microscopes, one that magnified 30 times. Looking through a thin slice of cork, he observed many tiny chambers that reminded him of monks’ cells in a monastery. Not long after that, the Dutch scientist Anton van [LEEUWENHOEK] used microscopes capable of magnifying 300 times and discovered an amazing world of single-celled life in a drop of pond water like in figure 1.10. He called the bacterial and protist cells he say “wee animalcules.” In 1839, the German biologists Matthias [SCHLEIDEN] and Theodor [SCHWANN], summarizing a large number of observations by themselves and others, concluded that all living organisms consist of cells. Their conclusion forms the basis of what has come to be known as the cell theory. Later, biologists added the idea that all cells come from other cells, the cell theory, one of the basic ideas in biology, is the foundation for understanding the reproduction and growth of all organisms. (28)
|
|
|
|
1. Even the simplest cell is incredibly complex, more intricate than a computer. The information that specifies what a cell is like – its detailed plan – is encoded in a long cable-like ________ called DNA (deoxyribonucleic acid). (28)
|
Even the simplest cell is incredibly complex, more intricate than a computer. The information that specifies what a cell is like – its detailed plan – is encoded in a long cable-like [MOLECULE] called DNA (deoxyribonucleic acid). (28)
|
|
|
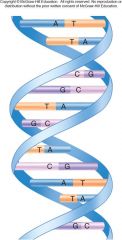
1. Researchers James ______ and Francis _____ discovered in 1953 that each DNA molecule is formed from two long chains of building blocks, called nucleotides, wound around each other. You can see in figure 1.11 that the two chains face each other, like two lines of people holding hands. The chains contain information in the same way this sentence does – as a sequence of letters. There are four different nucleotides in DNA (symbolized as A, T, C, and G in the figure), and the sequence in which they occur encodes the information. Specific sequences of several hundred to many thousand nucleotides make up a gene, a discrete unit of hereditary information. A gene might encode a particular protein or a different kind of unique molecule called RNA, or a gene might act to regulate other genes. (28) |
![Researchers James [WATSON] and Francis [CRICK] discovered in 1953 that each DNA molecule is formed from two long chains of building blocks, called nucleotides, wound around each other. You can see in figure 1.11 that the two chains face each other...](https://images.cram.com/images/upload-flashcards/51/28/31/6512831_m.jpg)
Researchers James [WATSON] and Francis [CRICK] discovered in 1953 that each DNA molecule is formed from two long chains of building blocks, called nucleotides, wound around each other. You can see in figure 1.11 that the two chains face each other, like two lines of people holding hands. The chains contain information in the same way this sentence does – as a sequence of letters. There are four different nucleotides in DNA (symbolized as A, T, C, and G in the figure), and the sequence in which they occur encodes the information. Specific sequences of several hundred to many thousand nucleotides make up a gene, a discrete unit of hereditary information. A gene might encode a particular protein or a different kind of unique molecule called RNA, or a gene might act to regulate other genes. (28) |
|
|
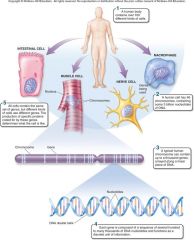
1. All organisms on earth encode their genes in strands of DNA. This prevalence of DNA led to the development of the gene theory. Illustrated in figure 1.12, the gene theory states that the ________ and RNA molecules encoded by an organism’s genes determine what it will be like. (28) |
![All organisms on earth encode their genes in strands of DNA. This prevalence of DNA led to the development of the gene theory. Illustrated in figure 1.12, the gene theory states that the [PROTEINS] and RNA molecules encoded by an organism’s gene...](https://images.cram.com/images/upload-flashcards/51/28/40/6512840_m.jpg)
All organisms on earth encode their genes in strands of DNA. This prevalence of DNA led to the development of the gene theory. Illustrated in figure 1.12, the gene theory states that the [PROTEINS] and RNA molecules encoded by an organism’s genes determine what it will be like. (28) |
|
|
|
1. The entire set of DNA instructions that specifies a cell is called its genome. The sequence of the human genome, 3 _______ nucleotides long, was decoded in ____, a triumph of scientific investigation. (28)
|
The entire set of DNA instructions that specifies a cell is called its genome. The sequence of the human genome, 3 [BILLION] nucleotides long, was decoded in [2001], a triumph of scientific investigation. (28)
|
|
|
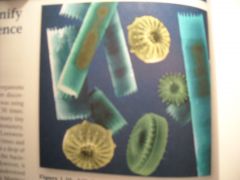
1. Figure 1.10 – Life in a drop of pond water – All _________ are composed of cells. Some organisms, including these protists, are single-celled, while others, such as plants, animals, and fungi, consist of many cells. (28) |
![Figure 1.10 – Life in a drop of pond water – All [ORGANISMS] are composed of cells. Some organisms, including these protists, are single-celled, while others, such as plants, animals, and fungi, consist of many cells. (28)](https://images.cram.com/images/upload-flashcards/51/28/55/6512855_m.jpg)
Figure 1.10 – Life in a drop of pond water – All [ORGANISMS] are composed of cells. Some organisms, including these protists, are single-celled, while others, such as plants, animals, and fungi, consist of many cells. (28) |
|
|
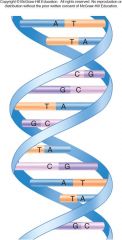
1. Figure 1.11 – Genes are made of DNA – Winding around each other like the rails of a spiral staircase, the two strands of a DNA molecule make a ______ _____. Because of its size and shape, the nucleotide represented by the letter A can only pair with the nucleotide represented by the letter T, and likewise G can only pair with C. (28) |
![Figure 1.11 – Genes are made of DNA – Winding around each other like the rails of a spiral staircase, the two strands of a DNA molecule make a [DOUBLE HELIX]. Because of its size and shape, the nucleotide represented by the letter A can only p...](https://images.cram.com/images/upload-flashcards/51/28/79/6512879_m.jpg)
Figure 1.11 – Genes are made of DNA – Winding around each other like the rails of a spiral staircase, the two strands of a DNA molecule make a [DOUBLE HELIX]. Because of its size and shape, the nucleotide represented by the letter A can only pair with the nucleotide represented by the letter T, and likewise G can only pair with C. (28) |
|
|
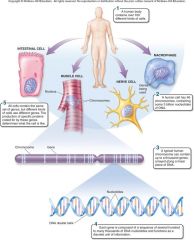
1. Figure 1.12 – The gene theory – The gene theory states that what an organism is like is determined in large measure by its genes. Here you see how the many kinds of cells in the body of each of us are determined by which genes are used in making each particular kind of ____. (29) |
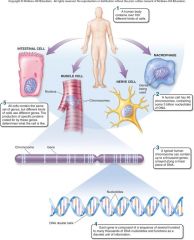
Figure 1.12 – The gene theory – The gene theory states that what an organism is like is determined in large measure by its genes. Here you see how the many kinds of cells in the body of each of us are determined by which genes are used in making each particular kind of [CELL]. (29) |
|
|
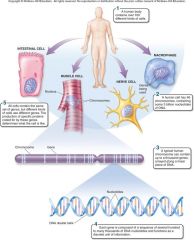
1. Figure 1.12 – The gene theory – (1) A human body contains over ___ different kinds of cells. (29) |
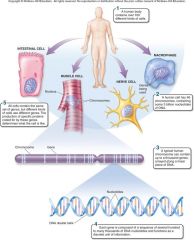
Figure 1.12 – The gene theory – (1) A human body contains over ___ different kinds of cells. (29) |
|
|
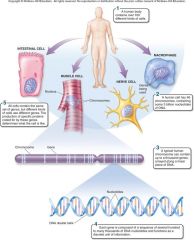
1. Figure 1.12 – The gene theory – (2) A human cell has 46 chromosomes, containing some 3 _______ nucleotides of DNA. (29) |
![Figure 1.12 – The gene theory – (2) A human cell has 46 chromosomes, containing some 3 [BILLION] nucleotides of DNA. (29)](https://images.cram.com/images/upload-flashcards/51/29/12/6512912_m.jpg)
Figure 1.12 – The gene theory – (2) A human cell has 46 chromosomes, containing some 3 [BILLION] nucleotides of DNA. (29) |
|
|
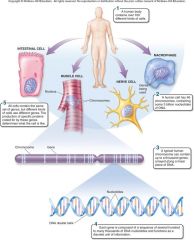
1. Figure 1.12 – The gene theory – (3) A typical human chromosome can contain up to a ________ genes, arrayed along a linear piece of DNA. (29) |
![Figure 1.12 – The gene theory – (3) A typical human chromosome can contain up to a [THOUSAND] genes, arrayed along a linear piece of DNA. (29)](https://images.cram.com/images/upload-flashcards/51/29/18/6512918_m.jpg)
Figure 1.12 – The gene theory – (3) A typical human chromosome can contain up to a [THOUSAND] genes, arrayed along a linear piece of DNA. (29) |
|
|
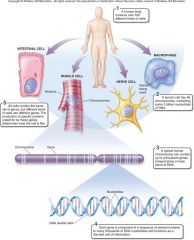
1. Figure 1.12 – The gene theory – (4) Each gene is composed of a sequence of several _______ to many _________ of DNA nucleotides and functions as a discrete unit of information. (29) |
![Figure 1.12 – The gene theory – (4) Each gene is composed of a sequence of several [HUNDRED] to many [THOUSANDS] of DNA nucleotides and functions as a discrete unit of information. (29)](https://images.cram.com/images/upload-flashcards/51/29/24/6512924_m.jpg)
Figure 1.12 – The gene theory – (4) Each gene is composed of a sequence of several [HUNDRED] to many [THOUSANDS] of DNA nucleotides and functions as a discrete unit of information. (29) |
|
|
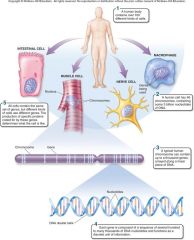
1. Figure 1.12 – The gene theory – (5) All cells contain the same set of genes, but different kinds of cells use different genes. The production of specific proteins _____ for by these genes determines what the cell is like. (29) |
![Figure 1.12 – The gene theory – (5) All cells contain the same set of genes, but different kinds of cells use different genes. The production of specific proteins [CODED] for by these genes determines what the cell is like. (29)](https://images.cram.com/images/upload-flashcards/51/29/30/6512930_m.jpg)
Figure 1.12 – The gene theory – (5) All cells contain the same set of genes, but different kinds of cells use different genes. The production of specific proteins [CODED] for by these genes determines what the cell is like. (29) |
|
|
|
1. The storage of __________ information in genes composed of DNA is common to all living things. (30)
|
The storage of [HEREDITARY] information in genes composed of DNA is common to all living things. (30)
|
|
|
|
1. The theory of heredity, first advanced by Gregor ______ in 1865, states that the genes of an organism are inherited as ________ units. A triumph of experimental science developed long before genes and DNA were understood. (30)
|
The theory of heredity, first advanced by Gregor [MENDEL] in 1865, states that the genes of an organism are inherited as [DISCRETE] units. A triumph of experimental science developed long before genes and DNA were understood. (30)
|
|
|
|
1. Soon after Mendel’s theory gave rise to the field of genetics, other biologists proposed what has come to be called the chromosomal theory of inheritance, which in its simplest form states that the _____ of Mendel’s theory are physically located on chromosomes and that it is because chromosomes are parceled out in a regular manner during ____________ that Mendel’s regular patterns of inheritance are seen. (30)
|
Soon after Mendel’s theory gave rise to the field of genetics, other biologists proposed what has come to be called the chromosomal theory of inheritance, which in its simplest form states that the [GENES] of Mendel’s theory are physically located on chromosomes and that it is because chromosomes are parceled out in a regular manner during [REPRODUCTION] that Mendel’s regular patterns of inheritance are seen. (30) |
|
|
1. In modern terms, the two theories (theory of heredity and theory of inheritance) state that genes are a _________ of a cell’s chromosomes (like the 23 pairs of human chromosomes you see in figure 1.13) and that the regular ___________ of these chromosomes during sexual reproduction is responsible for the pattern of inheritance we call Mendelian segregation. (30) |
![In modern terms, the two theories (theory of heredity and theory of inheritance) state that genes are a [COMPONENT] of a cell’s chromosomes (like the 23 pairs of human chromosomes you see in figure 1.13) and that the regular [DUPLICATION] of the...](https://images.cram.com/images/upload-flashcards/51/29/42/6512942_m.png)
In modern terms, the two theories (theory of heredity and theory of inheritance) state that genes are a [COMPONENT] of a cell’s chromosomes (like the 23 pairs of human chromosomes you see in figure 1.13) and that the regular [DUPLICATION] of these chromosomes during sexual reproduction is responsible for the pattern of inheritance we call Mendelian segregation. (30) |
|
|
|
1. The unity of life’s mechanisms among many related life-forms contrasts with the incredible _________ of living things that have _______ to fill the varied environments of Earth. (30)
|
The unity of life’s mechanisms among many related life-forms contrasts with the incredible [DIVERSITY] of living things that have [EVOLVED] to fill the varied environments of Earth. (30) |
|
|
|
1. Biologists sort organisms into kingdoms based on general _______________ in common. (30)
|
Biologists sort organisms into kingdoms based on general [CHARACTERISTICS] in common. (30)
|
|
|
|
1. In recent years, biologists have added a classification level above kingdoms, based on fundamental differences in ____ _________. (30)
|
In recent years, biologists have added a classification level above kingdoms, based on fundamental differences in [CELL STRUCTURE. (30)
|
|
|
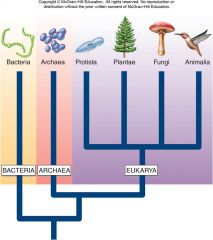
1. The six kingdoms are each now assigned into one of three great group called domains: ________, _______, and _______ (figure 1.14). (30) |
![The six kingdoms are each now assigned into one of three great group called domains: [BACTERIA], [ARCHAEA], and [EUKARYA] (figure 1.14). (30)](https://images.cram.com/images/upload-flashcards/51/29/48/6512948_m.jpg)
The six kingdoms are each now assigned into one of three great group called domains: [BACTERIA], [ARCHAEA], and [EUKARYA] (figure 1.14). (30) |
|
|
|
1. The theory of evolution, advanced by Charles Darwin in 1859, attributes the diversity of the living world to _______ _________. (30)
|
The theory of evolution, advanced by Charles Darwin in 1859, attributes the diversity of the living world to [NATURAL SELECTION]. (30)
|
|
|
|
1. Those _________ best able to respond to the challenges of living will leave more offspring, he (Charles Darwin) argued (natural selection), and thus their traits become more ______ in the population. (30)
|
Those [ORGANISMS] best able to respond to the challenges of living will leave more offspring, he (Charles Darwin) argued (natural selection), and thus their traits become more [COMMON] in the population. (30)
|
|
|
|
1. It is because the world offers diverse opportunities that it contains so many different ____-_____. (30)
|
It is because the world offers diverse opportunities that it contains so many different [LIFE-FORMS]. (30)
|
|
|
|
1. Today scientists can decipher the thousands of genes (the _______) of an organism. (30)
|
Today scientists can decipher the thousands of genes (the [GENOMES]) of an organism. (30)
|
|
|
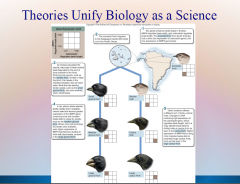
1. One of the great triumphs of science in the century and a half since Darwin is the detailed understanding of how Darwin’s theory of evolution is related to the ____ ______ – of how changes in life’s diversity can result from changes in __________ genes (figure 1.15). (30) |
![One of the great triumphs of science in the century and a half since Darwin is the detailed understanding of how Darwin’s theory of evolution is related to the [GENE THEORY] – of how changes in life’s diversity can result from changes in [IN...](https://images.cram.com/images/upload-flashcards/51/29/54/6512954_m.png)
One of the great triumphs of science in the century and a half since Darwin is the detailed understanding of how Darwin’s theory of evolution is related to the [GENE THEORY] – of how changes in life’s diversity can result from changes in [INDIVIDUAL] genes (figure 1.15). (30) |
|
|
|
1. Key Learning Outcome 1.9 – The theories uniting biology state that cellular organisms store __________ information in DNA. (30)
|
Key Learning Outcome 1.9 – The theories uniting biology state that cellular organisms store [HEREDITARY] information in DNA. (30)
|
|
|
|
1. Sometimes DNA ___________ occur, which when preserved result in evolutionary change. Today’s biological _________ is the product of a long evolutionary journey. (30)
|
Sometimes DNA [ALTERATIONS] occur, which when preserved result in evolutionary change. Today’s biological [DIVERSITY] is the product of a long evolutionary journey. (30)
|
|
|
|
1. Figure 1.13 – Human Chromosomes – The chromosomal theory of ___________ states that genes are located on chromosomes. This human karyotype (an ordering of chromosomes) shows banding patterns on chromosomes that represent clusters of _____. (30)
|
Figure 1.13 – Human Chromosomes – The chromosomal theory of [INHERITANCE] states that genes are located on chromosomes. This human karyotype (an ordering of chromosomes) shows banding patterns on chromosomes that represent clusters of [GENES]. (30)
|
|
|
|
1. Figure 1.14 – The three domains of life – Biologists categorize all ______ things into three overarching groups called domains: Bacteria, Archaea, and _______. Domain Bacteria contains the kingdom Bacteria, and domain Archaea contains the kingdom Archaea. (30)
|
Figure 1.14 – The three domains of life – Biologists categorize all [LIVING] things into three overarching groups called domains: Bacteria, Archaea, and [EUKARYA]. Domain Bacteria contains the kingdom Bacteria, and domain Archaea contains the kingdom Archaea. (30)
|
|
|
|
1. Domain Eukarya is composed of four more kingdoms: ________, _______, _____, and ________. (30)
|
Domain Eukarya is composed of four more kingdoms: [PROTISTA], [PLANTAE], [FUNGI], and [ANIMALIA]. (30)
|
|
|
|
1. Figure 1.15 – The theory of evolution – Darwin’s theory of evolution proposes that many forms of a ____ may exist among members of a __________ and that those members with a form better suited to their particular habitat will tend to reproduce more successfully, and so their ______ become more common in the population, a process Darwin dubbed “natural selection.” Here you see how this process is thought to have worked on two pivotal genes that helped generate the _________ of finches on the Galapagos Islands, visited by Darwin in 1831 on his round-the-world voyage on HMS Beagle.
|
Figure 1.15 – The theory of evolution – Darwin’s theory of evolution proposes that many forms of a [GENE] may exist among members of a [POPULATION] and that those members with a form better suited to their particular habitat will tend to reproduce more successfully, and so their [TRAITS] become more common in the population, a process Darwin dubbed “natural selection.” Here you see how this process is thought to have worked on two pivotal genes that helped generate the [DIVERSITY] of finches on the Galapagos Islands, visited by Darwin in 1831 on his round-the-world voyage on HMS Beagle.
|
|
|
|
1.1.1 Biology is the study of life. All living organisms share common _______________, but they are also diverse and are categorized into six groups called ________. (33)
|
Biology is the study of life. All living organisms share common [CHARACTERISTICS], but they are also diverse and are categorized into six groups called [KINGDOMS]. (33)
|
|
|
|
1.2.1 All living organisms share five basic properties: ________ ____________, metabolism, ___________, growth and reproduction, and ________. (33) |
1.2.1 All living organisms share five basic properties: [CELLULAR ORGANIZATION], metabolism, [HOMEOSTASIS], growth and reproduction, and [HEREDITY]. (33) |
|
|
|
Cellular ____________ indicates that all living organisms are composed of cells. (33)
|
Cellular [ORGANIZATION] indicates that all living organisms are composed of cells. (33)
|
|
|

Metabolism means that all living organisms, like the kingfisher shown here from figure 1.3, use ______. (33) |
![Metabolism means that all living organisms, like the kingfisher shown here from figure 1.3, use [ENERGY]. (33)](https://images.cram.com/images/upload-flashcards/51/29/60/6512960_m.png)
Metabolism means that all living organisms, like the kingfisher shown here from figure 1.3, use [ENERGY]. (33) |
|
|
|
Homeostasis is the process whereby all living organisms maintain stable ________ conditions. (33)
|
Homeostasis is the process whereby all living organisms maintain stable [INTERNAL] conditions. (33)
|
|
|
|
The properties of ______ ___ ____________ indicate that all living organisms grow in size and reproduce. (33)
|
The properties of [GROWTH AND REPRODUCTION] indicate that all living organisms grow in size and reproduce. (33)
|
|
|
|
The property of heredity describes how all living organisms possess _______ information in DNA that determines how each organism looks and functions, and this information is passed on to future ___________. (33)
|
The property of heredity describes how all living organisms possess [GENETIC] information in DNA that determines how each organism looks and functions, and this information is passed on to future [GENERATIONS]. (33)
|
|
|
|
1.3.1 Living organisms exhibit increasing levels of complexity within their cells (________ _____), within their bodies (__________ _____), and within ecosystems (____________ _____). (33) |
Living organisms exhibit increasing levels of complexity within their cells ([CELLULAR LEVEL]), within their bodies ([ORGANISMAL LEVEL]), and within ecosystems ([POPULATIONAL LEVEL]). (33) |
|
|
|
1.3.2 Novel properties that appear in each level of the hierarchy of life are called ________ __________. (33) |
Novel properties that appear in each level of the hierarchy of life are called [EMERGENT PROPERTIES]. (33)
|
|
|
|
1.4.1 Five themes emerge from the study of biology: _________, ___ ____ __ ______, ___________, _________ __________ ________, and ___________. These themes are used to examine the similarities and differences among organisms. (33) |
Five themes emerge from the study of biology: [EVOLUTION], [THE FLOW OF ENERGY], [COOPERATION], [STRUCTURE DETERMINES FUNCTION], and [HOMEOSTASIS]. These themes are used to examine the similarities and differences among organisms. (33) |
|
|
|
1.5.1 Mathematics uses general __________ to explain specific situations. (33) |
Mathematics uses general [PRINCIPLES] to explain specific situations. (33)
|
|
|
|
1.5.2 _________ reasoning is the process of using general principles to explain individual observations. _________ reasoning is the process of using specific observations to formulate general principles. (33) |
[DEDUCTIVE] reasoning is the process of using general principles to explain individual observations. [INDUCTIVE] reasoning is the process of using specific observations to formulate general principles. (33) |
|
|
|
1.6.1 Scientists observed a ________ of the ozone layer over Antarctica. Their scientific investigation of the “ozone hole” revealed that industrially produced ____ were responsible for the thinning of the ozone layer in the earth’s atmosphere (figure 1.6). (33) |
Scientists observed a [THINNING] of the ozone layer over Antarctica. Their scientific investigation of the “ozone hole” revealed that industrially produced [CFCs] were responsible for the thinning of the ozone layer in the earth’s atmosphere (figure 1.6). (33) |
|
|
|
1.7.1 Scientists use ____________ to formulate hypotheses. (33) |
1.7.1 Scientists use [OBSERVATIONS] to formulate hypotheses. (33) |
|
|
|
Hypotheses are possible ____________ that are used to form predictions. These predictions are tested ______________. (33)
|
Hypotheses are possible [EXPLANATIONS] that are used to form predictions. These predictions are tested [EXPERIMENTALLY]. (33)
|
|
|
|
Some hypotheses are ________ based on experimental results, while others are tentatively accepted. (33)
|
Some hypotheses are [REJECTED] based on experimental results, while others are tentatively accepted. (33)
|
|
|
|
1.7.2 Scientific investigations often use a series of ______, called the scientific process, to _____ a scientific question. |
1.7.2 Scientific investigations often use a series of [STAGES], called the scientific process, to [STUDY] a scientific question. |
|
|
|
The stages (in the scientific process) are ____________, forming __________, making ___________, _______, establishing ________, and drawing ___________. (33)
|
The stages (in the scientific process) are [OBSERVATIONS], forming [HYPOTHESES], making [PREDICTIONS], [TESTING], establishing [CONTROLS], and drawing [CONCLUSIONS]. (33)
|
|
|
|
The discovery of the hole in the ozone required careful ____________ of data collected from the atmosphere. Scientists proposed a __________ to explain what caused a decrease in the levels of ozone over the Antarctic. They then formed ___________ and tested the hypothesis against controls. (33) |
The discovery of the hole in the ozone required careful [OBSERVATIONS] of data collected from the atmosphere. Scientists proposed a [HYPOTHESIS] to explain what caused a decrease in the levels of ozone over the Antarctic. They then formed [PREDICTIONS] and tested the hypothesis against controls. (33)
|
|
|
|
1.8.1 __________ that hold up to testing over time are combined into statements called theories. Theories carry a higher degree of certainty, although no theory in science is ________. (33) |
[HYPOTHESES] that hold up to testing over time are combined into statements called theories. Theories carry a higher degree of certainty, although no theory in science is [ABSOLUTE]. (33)
|
|
|
|
1.8.2 Science can only _____ what can be tested experimentally, a hypothesis can only be established through science if it can be tested and potentially _________. (33) |
Science can only [STUDY] what can be tested experimentally, a hypothesis can only be established through science if it can be tested and potentially [DISPROVEN]. (33)
|
|
|
|
1.9.1 There are four unifying theories in biology: ____ theory, ____ theory, the theory of ________, and the theory of _________. (33) |
1.9.1 There are four unifying theories in biology: [CELL] theory, [GENE] theory, the theory of [HEREDITY], and the theory of [EVOLUTION]. (33) |
|
|
|
The cell theory states that all living organisms are composed of cells, which grow and _________ to form other cells. (33)
|
The cell theory states that all living organisms are composed of cells, which grow and [REPRODUCE] to form other cells. (33)
|
|
|
|
1.9.2 The gene theory states that long _________ of DNA carry instructions for producing cellular components. These instructions are encoded in the __________ sequences in the strands of DNA, like this section of DNA from figure 1.11. (33) |
1.9.2 The gene theory states that long [MOLECULES] of DNA carry instructions for producing cellular components. These instructions are encoded in the [NUCLEOTIDE] sequences in the strands of DNA, like this section of DNA from figure 1.11. (33) |
|
|
|
The nucleotides are organized into discrete _____ called genes, and the genes determine how an organism looks and _________. (33)
|
The nucleotides are organized into discrete [UNITS] called genes, and the genes determine how an organism looks and [FUNCTIONS]. (33)
|
|
|
|
1.9.3 The theory of ________ states that the genes of an organism are passed as discrete _____ from parent to offspring. (33) |
1.9.3 The theory of heredity states that the genes of an organism are passed as discrete [UNITS] from parent to offspring. (33) |
|
|
|
1.9.4 Organisms are organized into kingdoms based on similar _______________. (33) |
1.9.4 Organisms are organized into kingdoms based on similar [CHARACTERISTICS]. (33) |
|
|
|
The kingdoms are further organized into three major groups called _______ based on their ________ characteristics. (33) |
The kingdoms are further organized into three major groups called [DOMAINS] based on their [CELLULAR] characteristics. (33) |
|
|
|
The three domains are ________, _______, and _______. (33)
|
The three domains are [BACTERIA], [ARCHAEA], and [EUKARYA]. (33)
|
|
|
|
The theory of evolution states that _____________ in genes that are passed from parent to offspring result in changes in future generations. These changes lead to greater _________ among organisms over time. (33)
|
The theory of evolution states that [MODIFICATIONS] in genes that are passed from parent to offspring result in changes in future generations. These changes lead to greater [DIVERSITY] among organisms over time. (33) |
|
|
|
1.1.1 Biologists categorize all living things based on related characteristics into large groups called: (34)
a. Kingdoms. |
1.1.1 Biologists categorize all living things based on related characteristics into large groups called: (34)
a. Kingdoms. |
|
|
|
1.2.1a Living things can be distinguished from nonliving things because they have: (34)
a. Complexity. |
1.2.1a Living things can be distinguished from nonliving things because they have: (34)
c. Cellular organization. |
|
|
|
1.2.1b – In Fred Hoyle’s science fiction novel The Black Cloud, the earth is approached by a large interstellar cloud of gas. As the cloud orients around the sun, scientists discover that the cloud is feeding, absorbing the sun’s energy through the excitation of electrons in the outer energy levels of cloud molecules, a process similar to the photosynthesis that occurs on earth. Different portions of the cloud are isolated from each other by associations of ions created by this excitation. Electron currents pass between these sectors, much as they do on the surface of the human brain, and endow the cloud with self-awareness, memory and the ability to reason. Using electricity produced by static discharges, the cloud is able to communicate with human beings and to describe its history. The cloud tells our scientists that it once was smaller but has grown by absorbing molecules and energy from stars like our sun, on which it has been grazing. Eventually the cloud moves off in search of other stars. Is the cloud alive? Explain. (34) |
The cloud appears to be alive because it possesses several fundamental properties of life, including internal organization, energy transfer, growth, and possibly homeostasis. However, a key property of life that the cloud does not seem to possess is that of heredity. Although the cloud grows, it does not seem capable of reproducing offspring that carry characteristics passed on from the parent. Without this key characteristic, the cloud would not be considered alive. |
|
|
|
1.3.1 Living things are organized. Choose the answer that illustrates this organization and that is arranged from smallest to largest: (34)
a. Cell, atom, molecule, tissue, organelle, organ, organ system, organism, population, species, community, ecosystem. |
1.3.1 Living things are organized. Choose the answer that illustrates this organization and that is arranged from smallest to largest: (34)
b. Atom, molecule, organelle, cell, tissue, organ, organ system, organism, population, species, community, ecosystem. |
|
|
|
1.3.2 At each level in the hierarchy of living things, properties occur that were not present at the simpler levels. These properties are referred to as: (34)
a. Novelistic properties. |
1.3.2 At each level in the hierarchy of living things, properties occur that were not present at the simpler levels. These properties are referred to as: (34)
d. Emergent properties. |
|
|
|
1.3.2b Which of the following is not an emergent property: (34)
a. Metabolism |
1.3.2b Which of the following is not an emergent property: (34)
c. Cellular organization |
|
|
|
1.4.1 The five general biological themes include: (34)
a. Evolution, energy flow, competition, structure determines function, and homeostasis. |
1.4.1 The five general biological themes include: (34)
b. Evolution, energy flow, cooperation, structure determines function, and homeostasis. |
|
|
|
1.5.1 You notice that on cloudy days people often carry umbrellas, folded or in a case. You also not that when umbrellas are open, there are many car accidents. You conclude that open umbrellas cause car accidents. Explain the type of reasoning used to reach this conclusion and why it can sometimes be a problem. (34) |
You notice that on cloudy days people often carry umbrellas, folded or in a case. You also not that when umbrellas are open, there are many car accidents. You conclude that open umbrellas cause car accidents. Explain the type of reasoning used to reach this conclusion and why it can sometimes be a problem. (34)
This is an example of inductive reasoning. The problem is that people sometimes assume that because two items both occur at the same time, one of the events causes the other. In this case both events are caused by a third event that was not mentioned – the fact that it is raining. There is nothing wrong with the process of inductive reasoning, but do no assume that correlation (occurring together) implies causation. |
|
|
|
1.5.2 When you are trying to understand something new, you begin by observation and then put the observations together in a logical fashion to form a general principle. This method is called: (34)
a. Inductive reasoning. |
1.5.2 When you are trying to understand something new, you begin by observation and then put the observations together in a logical fashion to form a general principle. This method is called: (34)
a. Inductive reasoning. |
|
|
|
1.6.1 CFCs: (34)
a. Produce chlorine gas. |
1.6.1 CFCs: (34)
a. Produce chlorine gas. |
|
|
|
1.7.1 When trying to figure out explanations for observations, scientists construct a series of possible hypotheses. Then they make predictions and: (34)
a. Test each hypothesis, using appropriate controls, to determine which hypothesis is true. |
1.7.1 When trying to figure out explanations for observations, scientists construct a series of possible hypotheses. Then they make predictions and: (34)
b. Test each hypothesis, using appropriate controls, to rule out as many hypotheses as possible. |
|
|
|
1.7.2 Which of the following statements is correct regarding a hypothesis? (34) |
1.7.2 Which of the following statements is correct regarding a hypothesis? (34) |
|
|
|
1.8.1 St. John’s wort is an herb that has been used for hundreds of years as a remedy for mile depression. How might a modern-day scientist research its effectiveness? (34) |
St. John’s wort is an herb that has been used for hundreds of years as a remedy for mile depression. How might a modern-day scientist research its effectiveness? (34) |
|
|
|
1.8.2 The scientific method is: (34) |
1.8.2 The scientific method is: (34) |
|
|
|
1.9.1 Cell theory states that: (34) |
1.9.1 Cell theory states that: (34) |
|
|
|
1.9.2 The gene theory states that all the information that specifies what a cell is and what it does: (34) |
1.9.2 The gene theory states that all the information that specifies what a cell is and what it does: (34) |
|
|
|
1.9.3 The chromosomal theory of inheritance states that: (34) |
1.9.3 The chromosomal theory of inheritance states that: (34) |
|
|
|
1.9.4 The theory of evolution by natural selection was advanced in 1859 by: (34) |
1.9.4 The theory of evolution by natural selection was advanced in 1859 by: (34) |
|
|
|
The study of the properties of the substances that all things living and nonliving are created from. The branch of science that deals with the identification of the substances of which matter is composed; the investigation of their properties and the ways in which they interact, combine, and change; and the use of these processes to form new substances.
|
1. Chemistry (36)
|
|
|
|
Any substance in the universe that has mass and occupies space.
|
1. Matter (36)
|
|
|
|
A core (nucleus) of protons and neutrons surrounded by an orbiting cloud of electrons. The chemical behavior of an ____ is largely determined by the distribution of its electrons, particularly the number of electrons in its outermost level. |
1. Atoms (36)
|
|
|
|
A subatomic particle in the nucleus of an atom that carries a positive charge. The number of _______ determines the chemical character of the atom because it dictates the number of electrons orbiting the nucleus and available for chemical activity.
|
1. Protons (36)
|
|
|
|
A subatomic particle located within the nucleus of an atom. Similar to a proton in mass, but as its name implies, a _______ is neutral and possesses no charge.
|
1. Neutrons (36)
|
|
|
|
A subatomic particle with a negative electrical charge. The negative charge of one ________exactly balances the positive charge of one proton. _________ orbit the atom’s positively charged nucleus and determine its chemical properties.
|
1. Electron (36)
|
|
|
|
The number of protons in the nucleus of an atom. In an atom that does not bear an electric charge (that is, one that is not an ion), the ______ ______ is also equal to the number of electrons.
|
1. Atomic Number (36)
|
|
|
|
A substance that cannot be separated into different substances by ordinary chemical methods.
|
1. Element (36)
|
|
|
|
The ____ ______ of an atom consists of the combined mass of all of its protons and neutrons.
|
1. Mass Number (36)
|
|
|
|
The capacity to bring about change, to do work.
|
1. Energy (37)
|
|
|
|
The volume of space around a nucleus where an electron is most likely to be found.
|
1. Orbital (37)
|
|
|
|
An atom in which the number of electrons does not equal the number of protons. An ___ carries an electrical charge.
|
1. Ions (38)
|
|
|
|
An atom that has the same number of protons but different numbers of neutrons.
|
1. Isotopes (38)
|
|
|
|
The process by which a nucleus of an unstable atom loses energy by emitting ionizing radiation.
|
1. Radioactive Decay (aka Nuclear Decay or Radioactivity) (38)
|
|
|
|
A radioactive substance that is taken up and used by the body.
|
1. Tracer (38)
|
|
|
|
Any record of prehistoric life – generally taken to mean older than 10,000 years.
|
1. Fossil (39)
|
|
|
|
A widely employed method of dating fossils less than 50,000 years old is the carbon-14 (14C) _____________ ______. Any method of determining the age of earth materials or objects of organic origin based on measurement of either short-lived radioactive elements or the amount of a long-lived radioactive element plus its decay product.
|
1. Radioisotopic Dating (39)
|
|
|
|
The length of time it takes for half of a radioactive substance to decay.
|
1. Half-Life (39)
|
|
|
|
The smallest unit of a compound that displays the properties of that compound.
|
1. Molecule (40)
|
|
|
|
The force holding two atoms together. The force can result from the attraction of opposite charges (ionic bond) or from the sharing of one or more pairs of electrons (a covalent bond).
|
1. Chemical Bond (40)
|
|
|
|
A chemical bond formed between ions as a result of the attraction of opposite electrical charges.
|
1. Ionic Bonds (40)
|
|
|
|
A chemical bond formed by the sharing of one or more pairs of electrons.
|
1. Covalent Bonds (41)
|
|
|
|
A molecule with positively and negatively charged ends. One portion of a _____ ________ attracts electrons more strongly than another portion, with the result that the molecule has electron-rich (-) and electron-poor (+) regions, giving it magnet-like positive and negative poles. Water is one of the most _____ _________ known.
|
1. Polar Molecules (41)
|
|
|
|
Molecules that don’t exhibit a large difference in electronegativities of its atoms, like the carbon-hydrogen bonds of methane, are called ________ _________ and contain ________ covalent bonds.
|
1. Nonpolar Molecules (41)
|
|
|
|
A molecular force formed by the attraction of the partial positive charge of one hydrogen atom of a water molecule with the partial negative charge of the oxygen atom of another.
|
1. Hydrogen Bond (42)
|
|
|
|
The molecular attraction between the surfaces of like bodies in contact, such as that between water molecules and other water molecules.
|
1. Cohesion (44)
|
|
|
|
The molecular attraction exerted between the surfaces of unlike bodies in contact, as water molecules to the walls of the narrow tubes that occur in plants.
|
1. Adhesion (44)
|
|
|
|
Describes polar molecules, which form hydrogen bonds with water and therefore are soluble in water.
|
1. Hydrophilic (44)
|
|
|
|
Refers to polar molecules that dissolve in water and are surrounded by a hydration shell.
|
1. Soluble (44)
|
|
|
|
Describes nonpolar molecules, which do not form hydrogen bonds with water and therefore are not soluble in water.
|
1. Hydrophobic (44)
|
|
|
|
The positively charged ___ of ________, H+; formed by removal of the electron from atomic ________ and found in the form of hydronium ___ in all aqueous solutions of acids.
|
1. Hydrogen Ion (45)
|
|
|
|
A negatively charged particle of _________ (OH-) of any base in a water solution.
|
1. Hydroxide Ion (45)
|
|
|
|
The process of spontaneous ion formation. One of the principal ways that radiation, such as charged particles and X rays, transfers its energy to matter. In chemistry, __________ often occurs in a liquid solution.
|
1. Ionization (45)
|
|
|
|
Any substance that dissociates to form H+ ions when dissolved in water. Having a pH value less than 7.
|
1. Acid (45)
|
|
|
|
Any substance that combines with H+ ions thereby reducing the H+ ion concentration of a solution. Having a pH value above 7.
|
1. Base (45)
|
|
|
|
Rainfall made sufficiently acidic by atmospheric pollution that it causes environmental harm, typically to forests and lakes. The main cause is the industrial burning of coal and other fossil fuels, the waste gases from which contain sulfur and nitrogen oxides, which combine with atmospheric water to form acids.
|
1. Acid Rain (46)
|
|
|
|
A substance that takes up or releases hydrogen ions (H+) to maintain the pH within a certain range.
|
1. Buffer (47)
|
|
|
|
1. Atoms: Chemistry – study of the properties of __________. |
Atoms: Chemistry – study of the properties of [SUBSTANCES]. |
|
|
|
1. Atoms: Matter – any substance that has ____ and occupies _____.
|
Atoms: Matter – any substance that has [MASS] and occupies [SPACE]. |
|
|
|
1. Atoms: Atoms – smallest particle into which a substance can be divided and still retain its ________ properties.
|
Atoms: Atoms – smallest particle into which a substance can be divided and still retain its [CHEMICAL] properties. |
|
|
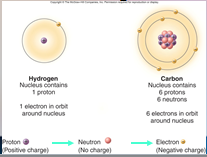
Atoms: Protons – Subatomic particle with a ________ charge and are part of a very dense _______ of an atom. |
![Atoms: Protons – Subatomic particle with a [POSITIVE] charge and are part of a very dense [NUCLEUS] of an atom.](https://images.cram.com/images/upload-flashcards/54/35/96/6543596_m.png)
Atoms: Protons – Subatomic particle with a [POSITIVE] charge and are part of a very dense [NUCLEUS] of an atom. |
|
|
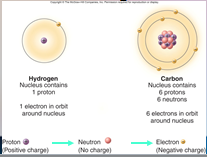
Atoms: Neutron – Subatomic particle without a charge (_______) and are part of a very dense nucleus of an ____. |
![Atoms: Neutron – Subatomic particle without a charge ([NEUTRAL]) and are part of a very dense nucleus of an [ATOM].](https://images.cram.com/images/upload-flashcards/54/36/02/6543602_m.png)
Atoms: Neutron – Subatomic particle without a charge ([NEUTRAL]) and are part of a very dense nucleus of an [ATOM]. |
|
|
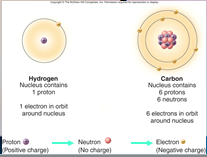
Atoms: Electron – Subatomic particle with a ________ charge that ______ the core nucleus. |
![Atoms: Electron – Subatomic particle with a [NEGATIVE] charge that [ORBITS] the core nucleus.](https://images.cram.com/images/upload-flashcards/54/36/11/6543611_m.png)
Atoms: Electron – Subatomic particle with a [NEGATIVE] charge that [ORBITS] the core nucleus. |
|
|

1. Atoms: Atoms – What does an atom really look like? |

Atoms: Atoms – What does an atom really look like? |
|
|
Atoms: Atomic Number – number of _______ in the nucleus. |
![Atoms: Atomic Number – number of [PROTONS] in the nucleus.](https://images.cram.com/images/upload-flashcards/54/36/35/6543635_m.png)
Atoms: Atomic Number – number of [PROTONS] in the nucleus. |
|
|
Atoms: Element – atoms with the same ______ number that have the same chemical properties. |
![Atoms: Element – atoms with the same [ATOMIC] number that have the same chemical properties.](https://images.cram.com/images/upload-flashcards/54/36/47/6543647_m.png)
Atoms: Element – atoms with the same [ATOMIC] number that have the same chemical properties. |
|
|
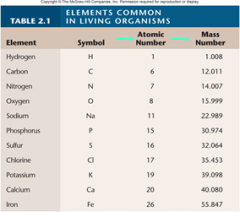
1. Atoms: Mass Number – number of _______ and ________. |
![Atoms: Mass Number – number of [PROTONS] and [NEUTRONS].](https://images.cram.com/images/upload-flashcards/54/36/56/6543656_m.png)
Atoms: Mass Number – number of [PROTONS] and [NEUTRONS]. |
|
|
1. Atoms: Energy – ability to ____. |
![Atoms: Energy – ability to [WORK].](https://images.cram.com/images/upload-flashcards/54/37/07/6543707_m.png)
Atoms: Energy – ability to [WORK]. |
|
|
1. Atoms: Potential Energy – energy of position (drive ________ _________). |
![Atoms: Potential Energy – energy of position (drive [CHEMICAL REACTIONS]).](https://images.cram.com/images/upload-flashcards/54/37/16/6543716_m.png)
Atoms: Potential Energy – energy of position (drive [CHEMICAL REACTIONS]). |
|
|
1. Atoms: Energy levels are called ________ ______. |
![Atoms: Energy levels are called [ELECTRON SHELLS].](https://images.cram.com/images/upload-flashcards/54/37/64/6543764_m.png)
Atoms: Energy levels are called [ELECTRON SHELLS]. |
|
|
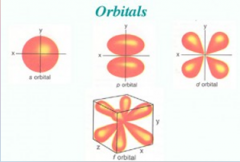
1. Atoms: Orbital – volume of space around the nucleus where an ________ is most likely to be found. |
![Atoms: Orbital – volume of space around the nucleus where an [ELECTRON] is most likely to be found.](https://images.cram.com/images/upload-flashcards/54/37/73/6543773_m.png)
Atoms: Orbital – volume of space around the nucleus where an [ELECTRON] is most likely to be found. |
|
|
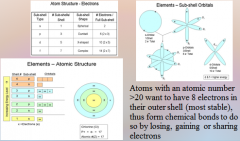
Electron Shells: Each electron shell has a specific number of ________. |
![Electron Shells: Each electron shell has a specific number of [ORBITALS].](https://images.cram.com/images/upload-flashcards/54/37/22/6543722_m.png)
Electron Shells: Each electron shell has a specific number of [ORBITALS]. |
|
|
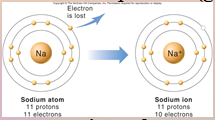
1. Ions and Isotopes: Ion – atoms in which the number of electrons does not equal the number of _______ (gain or loss of electron). |
![Ions and Isotopes: Ion – atoms in which the number of electrons does not equal the number of [PROTONS] (gain or loss of electron).](https://images.cram.com/images/upload-flashcards/54/37/31/6543731_m.png)
Ions and Isotopes: Ion – atoms in which the number of electrons does not equal the number of [PROTONS] (gain or loss of electron). |
|
|

Ions and Isotopes: Isotopes – same number of protons, but different numbers of ________. |
![Ions and Isotopes: Isotopes – same number of protons, but different numbers of [NEUTRONS].](https://images.cram.com/images/upload-flashcards/54/37/43/6543743_m.png)
Ions and Isotopes: Isotopes – same number of protons, but different numbers of [NEUTRONS]. |
|
|
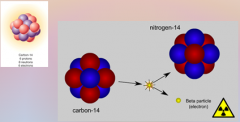
Ions and Isotopes: Radioactive Decay – _______ tends to break up into particles with lower atomic number. |
![Ions and Isotopes: Radioactive Decay – [NUCLEUS] tends to break up into particles with lower atomic number.](https://images.cram.com/images/upload-flashcards/54/37/94/6543794_m.png)
Ions and Isotopes: Radioactive Decay – [NUCLEUS] tends to break up into particles with lower atomic number. |
|
|
1. Ions and Isotopes: Tracer – radioactive substance that is taken up and used by the ____. |
![Ions and Isotopes: Tracer – radioactive substance that is taken up and used by the [BODY].](https://images.cram.com/images/upload-flashcards/54/38/12/6543812_m.png)
Ions and Isotopes: Tracer – radioactive substance that is taken up and used by the [BODY]. |
|
|
Ions and Isotopes: Tracers are helpful in identifying _________ tumors. |
![Ions and Isotopes: Tracers are helpful in identifying [CANCEROUS] tumors.](https://images.cram.com/images/upload-flashcards/54/38/21/6543821_m.png)
Ions and Isotopes: Tracers are helpful in identifying [CANCEROUS] tumors. |
|
|

Ions and Isotopes: Fossils – created by the remains, footprints, or other traces of _________ that become buried in sand or sediment. |
![Ions and Isotopes: Fossils – created by the remains, footprints, or other traces of [ORGANISMS] that become buried in sand or sediment.](https://images.cram.com/images/upload-flashcards/54/38/42/6543842_m.png)
Ions and Isotopes: Fossils – created by the remains, footprints, or other traces of [ORGANISMS] that become buried in sand or sediment. |
|
|

Ions and Isotopes: Radioisotopic dating – method of dating fossils less than 50,000 years old using ______-__. |
![Ions and Isotopes: Radioisotopic dating – method of dating fossils less than 50,000 years old using [CARBON-14].](https://images.cram.com/images/upload-flashcards/54/38/48/6543848_m.png)
Ions and Isotopes: Radioisotopic dating – method of dating fossils less than 50,000 years old using [CARBON-14]. |
|
|
Ions and Isotopes: After an organism dies carbon-14 decays, but no additional carbon-12 is taken into the ____. |
![Ions and Isotopes: After an organism dies carbon-14 decays, but no additional carbon-12 is taken into the [BODY].](https://images.cram.com/images/upload-flashcards/54/38/60/6543860_m.png)
Ions and Isotopes: After an organism dies carbon-14 decays, but no additional carbon-12 is taken into the [BODY]. |
|
|
Ions and Isotopes: The ratio of carbon-14 to carbon-12 decreases by ½ every _,___ years (half-life). |
![Ions and Isotopes: The ratio of carbon-14 to carbon-12 decreases by ½ every [5,730] years (half-life).](https://images.cram.com/images/upload-flashcards/54/38/78/6543878_m.png)
Ions and Isotopes: The ratio of carbon-14 to carbon-12 decreases by ½ every [5,730] years (half-life). |
|
|
|
1. Molecules: Molecule – a group of _____ held together by energy.
|
Molecules: Molecule – a group of [ATOMS] held together by energy. |
|
|
|
1. Molecules: Chemical Bond – energy or force holding two atoms together (_____ bonds,________ bonds, ________ bonds, ___ ___ _____ ______).
|
Molecules: Chemical Bond – energy or force holding two atoms together ([IONIC] bonds, [COVALENT] bonds, [HYDROGEN] bonds, [VAN DER WAALS FORCES]). |
|
|
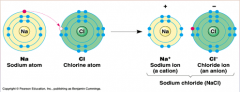
Molecules: Ionic bonds – atoms are attracted to each other by ________ electrical charges. |
![Molecules: Ionic bonds – atoms are attracted to each other by [OPPOSITE] electrical charges.](https://images.cram.com/images/upload-flashcards/54/38/90/6543890_m.png)
Molecules: Ionic bonds – atoms are attracted to each other by [OPPOSITE] electrical charges. |
|
|
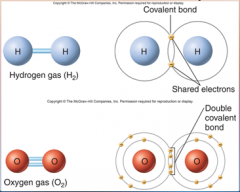
Molecules: Covalent bonds – between two atoms when they _____ electrons. |
![Molecules: Covalent bonds – between two atoms when they [SHARE] electrons.](https://images.cram.com/images/upload-flashcards/54/38/99/6543899_m.png)
Molecules: Covalent bonds – between two atoms when they [SHARE] electrons. |
|
|
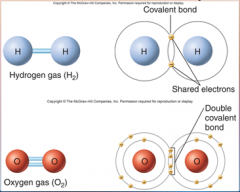
1. Molecules: Covalent bonds – between two _____ when they share electrons. |
![Molecules: Covalent bonds – between two [ATOMS] when they share electrons.](https://images.cram.com/images/upload-flashcards/54/39/17/6543917_m.png)
Molecules: Covalent bonds – between two [ATOMS] when they share electrons. |
|
|

1. Molecules: Polar molecules – have ________ and ________ ends “poles.” |
![Molecules: Polar molecules – have [POSITIVE] and [NEGATIVE] ends “poles.”](https://images.cram.com/images/upload-flashcards/54/39/26/6543926_m.png)
Molecules: Polar molecules – have [POSITIVE] and [NEGATIVE] ends “poles.” |
|
|

Molecules: Nonpolar molecules – don’t exhibit a large difference in _________________ of its atoms. |
![Molecules: Nonpolar molecules – don’t exhibit a large difference in [ELECTRONEGATIVITIES] of its atoms.](https://images.cram.com/images/upload-flashcards/54/39/32/6543932_m.png)
Molecules: Nonpolar molecules – don’t exhibit a large difference in [ELECTRONEGATIVITIES] of its atoms. |
|
|
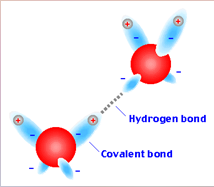
Molecules: Hydrogen bond – positive end of one polar molecule is _________ to the negative end of another. |
![Molecules: Hydrogen bond – positive end of one polar molecule is [ATTRACTED] to the negative end of another.](https://images.cram.com/images/upload-flashcards/54/39/41/6543941_m.png)
Molecules: Hydrogen bond – positive end of one polar molecule is [ATTRACTED] to the negative end of another. |
|
|
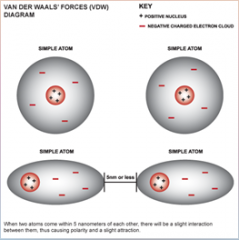
1. Molecules: Van der Waals Forces – ___-___________ attractive force that occurs when two atoms are very close to one another. |
![Molecules: Van der Waals Forces – [NON-DIRECTIONAL] attractive force that occurs when two atoms are very close to one another.](https://images.cram.com/images/upload-flashcards/54/39/50/6543950_m.png)
Molecules: Van der Waals Forces – [NON-DIRECTIONAL] attractive force that occurs when two atoms are very close to one another. |
|
|
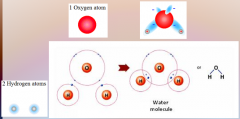
1. _____: 2H2 + O2 → 2H2O |
![[WATER]: 2H2 + O2 → 2H2O](https://images.cram.com/images/upload-flashcards/54/39/59/6543959_m.png)
[WATER]: 2H2 + O2 → 2H2O |
|
|

1. Water: ________ bonds give water unique properties. |
![Water: [HYDROGEN] bonds give water unique properties.](https://images.cram.com/images/upload-flashcards/54/39/68/6543968_m.png)
Water: [HYDROGEN] bonds give water unique properties. |
|
|
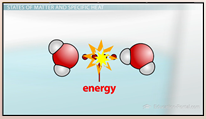
1. Water: Heat Storage – Many hydrogen bonds that water molecules form with one another need a large input of thermal ______ to disrupt the organization of liquid water and raise its ___________. |
![Water: Heat Storage – Many hydrogen bonds that water molecules form with one another need a large input of thermal [ENERGY] to disrupt the organization of liquid water and raise its [TEMPERATURE].](https://images.cram.com/images/upload-flashcards/54/39/77/6543977_m.png)
Water: Heat Storage – Many hydrogen bonds that water molecules form with one another need a large input of thermal [ENERGY] to disrupt the organization of liquid water and raise its [TEMPERATURE]. |
|
|
Water: ________ ____ – the energy needed to raise the temperature of 1g of a substance 1˚C. |
![Water: [SPECIFIC HEAT] – the energy needed to raise the temperature of 1g of a substance 1˚C.](https://images.cram.com/images/upload-flashcards/54/39/83/6543983_m.png)
Water: [SPECIFIC HEAT] – the energy needed to raise the temperature of 1g of a substance 1˚C. |
|
|
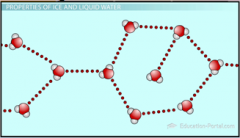
Water: Ice Formation – A lattice of hydrogen bonds assume a _______-____ structure, forming a solid. |
![Water: Ice Formation – A lattice of hydrogen bonds assume a [CRYSTAL-LIKE] structure, forming a solid.](https://images.cram.com/images/upload-flashcards/54/39/95/6543995_m.png)
Water: Ice Formation – A lattice of hydrogen bonds assume a [CRYSTAL-LIKE] structure, forming a solid. |
|
|
1. Water: High heat of vaporization – High ____________ causes many hydrogen bonds to break in water with the result that the liquid is changed into vapor. |
![Water: High heat of vaporization – High [TEMPERATURES] causes many hydrogen bonds to break in water with the result that the liquid is changed into vapor.](https://images.cram.com/images/upload-flashcards/54/40/07/6544007_m.png)
Water: High heat of vaporization – High [TEMPERATURES] causes many hydrogen bonds to break in water with the result that the liquid is changed into vapor. |
|
|
Water: Cohesion – the attraction of between a _____ molecule to another _____ molecule. |
![Water: Cohesion – the attraction of between a [POLAR] molecule to another [POLAR] molecule.](https://images.cram.com/images/upload-flashcards/54/40/19/6544019_m.png)
Water: Cohesion – the attraction of between a [POLAR] molecule to another [POLAR] molecule. |
|
|
Water: Adhesion – A polar molecule is attracted to the polar molecule of another _________. |
![Water: Adhesion – A polar molecule is attracted to the polar molecule of another [SUBSTANCE].](https://images.cram.com/images/upload-flashcards/54/41/12/6544112_m.png)
Water: Adhesion – A polar molecule is attracted to the polar molecule of another [SUBSTANCE]. |
|
|
1. Water: High Polarity – Water molecules in solution always tend to form the maximum number of hydrogen _____ possible. |
![Water: High Polarity – Water molecules in solution always tend to form the maximum number of hydrogen [BONDS] possible.](https://images.cram.com/images/upload-flashcards/54/41/24/6544124_m.png)
Water: High Polarity – Water molecules in solution always tend to form the maximum number of hydrogen [BONDS] possible. |
|
|
1. Water: Hydrophilic – “_____ ______.” |
![Water: Hydrophilic – “[WATER LOVING].”](https://images.cram.com/images/upload-flashcards/54/41/75/6544175_m.png)
Water: Hydrophilic – “[WATER LOVING].” |
|
|
Water: Soluble – ______ of water form around all polar molecules and the polar molecules ________ in water. |
![Water: Soluble – [SHELLS] of water form around all polar molecules and the polar molecules [DISSOLVE] in water.](https://images.cram.com/images/upload-flashcards/54/41/84/6544184_m.png)
Water: Soluble – [SHELLS] of water form around all polar molecules and the polar molecules [DISSOLVE] in water. |
|
|

1. Water: High Polarity – Water molecules in solution always tend to form the _______ number of hydrogen bonds possible. |
![Water: High Polarity – Water molecules in solution always tend to form the [MAXIMUM] number of hydrogen bonds possible.](https://images.cram.com/images/upload-flashcards/54/41/96/6544196_m.png)
Water: High Polarity – Water molecules in solution always tend to form the [MAXIMUM] number of hydrogen bonds possible. |
|
|

1. Water: ___________ – “water fearing.” |
![Water: [HYDROPHOBIC] – “water fearing.”](https://images.cram.com/images/upload-flashcards/54/42/02/6544202_m.png)
Water: [HYDROPHOBIC] – “water fearing.” |
|
|

1. Water: ________ molecules are forced into association with one another, crowded together to minimize their disruption of the ________ bonding of water. |
![Water: [NONPOLAR] molecules are forced into association with one another, crowded together to minimize their disruption of the [HYDROGEN] bonding of water.](https://images.cram.com/images/upload-flashcards/54/42/14/6544214_m.png)
Water: [NONPOLAR] molecules are forced into association with one another, crowded together to minimize their disruption of the [HYDROGEN] bonding of water. |
|
|
Water Ionizes: Hydrogen Ion (H+) – positively charged hydrogen ion due to the loss of a(n) ________. |
![Water Ionizes: Hydrogen Ion (H+) – positively charged hydrogen ion due to the loss of a(n) [ELECTRON].](https://images.cram.com/images/upload-flashcards/54/42/26/6544226_m.png)
Water Ionizes: Hydrogen Ion (H+) – positively charged hydrogen ion due to the loss of a(n) [ELECTRON]. |
|
|
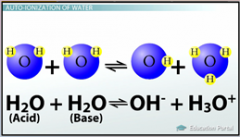
Water Ionizes: Hydroxide Ion (OH-) – __________ charged ion due to the gain of an electron. |
![Water Ionizes: Hydroxide Ion (OH-) – [NEGATIVELY] charged ion due to the gain of an electron.](https://images.cram.com/images/upload-flashcards/54/42/32/6544232_m.png)
Water Ionizes: Hydroxide Ion (OH-) – [NEGATIVELY] charged ion due to the gain of an electron. |
|
|
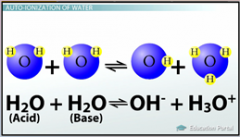
1. Water Ionizes: Ionization – spontaneous ion _________. |
![Water Ionizes: Ionization – spontaneous ion [FORMATION].](https://images.cram.com/images/upload-flashcards/54/42/41/6544241_m.png)
Water Ionizes: Ionization – spontaneous ion [FORMATION]. |
|
|
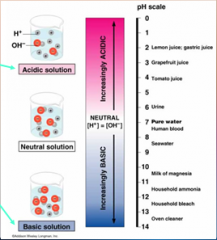
1. Water Ionizes: pH – expression of the _____________ of H+ in solution. |
![Water Ionizes: pH – expression of the [CONCENTRATION] of H+ in solution.](https://images.cram.com/images/upload-flashcards/54/42/56/6544256_m.png)
Water Ionizes: pH – expression of the [CONCENTRATION] of H+ in solution. |
|
|
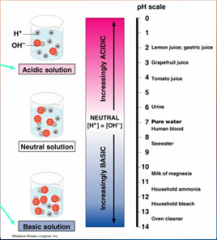
Water Ionizes: Acids – substance that ___________ in water to increase the concentration of H+ in water. |
![Water Ionizes: Acids – substance that [DISSOCIATED] in water to increase the concentration of H+ in water.](https://images.cram.com/images/upload-flashcards/54/42/71/6544271_m.png)
Water Ionizes: Acids – substance that [DISSOCIATED] in water to increase the concentration of H+ in water. |
|
|
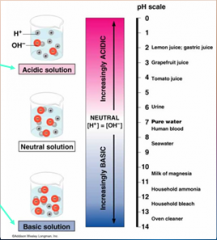
1. Water Ionizes: Bases – substance that ________ with H+ in water. |
![Water Ionizes: Bases – substance that [COMBINES] with H+ in water.](https://images.cram.com/images/upload-flashcards/54/42/77/6544277_m.png)
Water Ionizes: Bases – substance that [COMBINES] with H+ in water. |
|
|
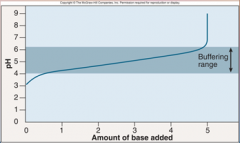
1. Water Ionizes: Buffers – substance that takes up or releases hydrogen ions into solution as the hydrogen ion concentration of the solution _______. Resist change in pH. |
![Water Ionizes: Buffers – substance that takes up or releases hydrogen ions into solution as the hydrogen ion concentration of the solution [CHANGES]. Resist change in pH.](https://images.cram.com/images/upload-flashcards/54/42/92/6544292_m.png)
Water Ionizes: Buffers – substance that takes up or releases hydrogen ions into solution as the hydrogen ion concentration of the solution [CHANGES]. Resist change in pH. |
|
|
|
1. Biology is the science of life, and all life, in fact even all _______, is made of __________. (36)
|
Biology is the science of life, and all life, in fact even all [NONLIFE], is made of [SUBSTANCES]. (36) |
|
|
|
1. Chemistry is the study of the __________ of these substances. So, while it may seem tedious or unrelated to examine chemistry in a biology text, it is essential. (36)
|
Chemistry is the study of the [PROPERTIES] of these substances. So, while it may seem tedious or unrelated to examine chemistry in a biology text, it is essential. (36) |
|
|
|
1. Organisms are ________ machines (figure 2.1), and to understand them we must learn a little chemistry. (36)
|
Organisms are [CHEMICAL] machines (figure 2.1), and to understand them we must learn a little chemistry. (36) |
|
|
|
1. Any substance in the universe that has ____ and occupies _____ is defined as matter. (36)
|
Any substance in the universe that has [MASS] and occupies [SPACE] is defined as matter. (36) |
|
|
|
1. All matter is composed of extremely small particles called _____. (36) |
All matter is composed of extremely small particles called [ATOMS]. (36) |
|
|
|
1. An atom is the smallest particle into which a substance can be _______ and still retain its chemical properties. (36) |
An atom is the smallest particle into which a substance can be [DIVIDED] and still retain its chemical properties. (36) |
|
|
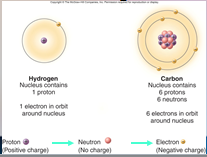
1. Every atom has the same basic _________ you see in figure 2.2. (36) |
![Every atom has the same basic [STRUCTURE] you see in figure 2.2. (36)](https://images.cram.com/images/upload-flashcards/54/43/76/6544376_m.png)
Every atom has the same basic [STRUCTURE] you see in figure 2.2. (36) |
|
|
|
1. At the center of every ____ is a small, very dense nucleus formed of two types of _________ particles, protons (illustrated by purple balls) and neutrons (the pink balls). Whizzing around the core is an orbiting cloud of a third kind of subatomic particle, the electron (depicted by yellow balls on concentric rings). (36) |
At the center of every [ATOM] is a small, very dense nucleus formed of two types of [SUBATOMIC] particles, protons (illustrated by purple balls) and neutrons (the pink balls). Whizzing around the core is an orbiting cloud of a third kind of subatomic particle, the electron (depicted by yellow balls on concentric rings). (36) |
|
|
|
1. ________ have no electrical charge, whereas _______ have a positive charge and _________ have a negative one. (36)
|
[NEUTRONS] have no electrical charge, whereas [PROTONS] have a positive charge and [ELECTRONS] have a negative one. (36) |
|
|
|
1. In a typical ____, there is an orbiting electron for every proton in the nucleus. The electron’s negative charge balances the proton’s positive charge, so the atom is electrically _______. (36)
|
In a typical [ATOM], there is an orbiting electron for every proton in the nucleus. The electron’s negative charge balances the proton’s positive charge, so the atom is electrically [NEUTRAL]. (36) |
|
|
|
1. An atom is typically described by the number of _______ in its nucleus or by the overall ____ of the atom. The terms mass and weight are often used interchangeably, but they have slightly different meaning. (36)
|
An atom is typically described by the number of [PROTONS] in its nucleus or by the overall [MASS] of the atom. The terms mass and weight are often used interchangeably, but they have slightly different meaning. (36) |
|
|
|
1. Mass refers to the ______ of a substance, whereas weight refers to the force _______ exerts on a substance. Hence, an object has the same mass whether it is on the earth or the moon, but its weight will be greater on the earth, because the earth’s gravitational force is greater than the moon’s. For example, an astronaut weighting 180 pounds on Earth will weigh about 30 pounds on the moon. He didn’t lose any significant mass during his flight to the moon; there is just less gravitational pull on his mass. (36)
|
Mass refers to the [AMOUNT] of a substance, whereas weight refers to the force [GRAVITY] exerts on a substance. Hence, an object has the same mass whether it is on the earth or the moon, but its weight will be greater on the earth, because the earth’s gravitational force is greater than the moon’s. For example, an astronaut weighting 180 pounds on Earth will weigh about 30 pounds on the moon. He didn’t lose any significant mass during his flight to the moon; there is just less gravitational pull on his mass. (36)
|
|
|
|
1. The number of protons in the _______ of an atom is called the atomic number. For example, the atomic number of carbon is 6 because it has six protons. (36)
|
The number of protons in the [NUCLEUS] of an atom is called the atomic number. For example, the atomic number of carbon is 6 because it has six protons. (36)
|
|
|
|
1. Atoms with the same atomic number (that is, the same number of protons) have the same ________ __________ and are said to belong to the same element. (36)
|
Atoms with the same atomic number (that is, the same number of protons) have the same [CHEMICAL PROPERTIES] and are said to belong to the same element. (36)
|
|
|
|
1. An element is any substance that cannot be ______ ____ into any other substance by ordinary chemical means. (36)
|
An element is any substance that cannot be [BROKEN DOWN] into any other substance by ordinary chemical means. (36)
|
|
|
|
1. Neutrons are similar to protons in ____, and the number of protons and neutrons in the nucleus of an atom is called the ____ ______. (36)
|
Neutrons are similar to protons in [MASS], and the number of protons and neutrons in the nucleus of an atom is called the [MASS NUMBER]. (36)
|
|
|
|
1. A carbon atom that has six _______ and six ________ has a mass number of 12. (36)
|
A carbon atom that has six [PROTONS] and six [NEUTRONS] has a mass number of 12. (36)
|
|
|
|
1. An electron’s contribution to the overall ____ of an atom is negligible. (36)
|
An electron’s contribution to the overall [MASS] of an atom is negligible. (36)
|
|
|
|
1. The atomic numbers and mass numbers of some of the most ______ elements on Earth are shown in table 2.1. (36)
|
The atomic numbers and mass numbers of some of the most [COMMON] elements on Earth are shown in table 2.1. (36)
|
|
|
|
1. Figure 2.1 – Replacing electrolytes. During extreme exercise, athlete will often consume drinks that contain “electrolytes,” chemical such as calcium, potassium, and sodium that play an important role in muscle contraction. Electrolytes can also be depleted in other types of ___________. (36)
|
Figure 2.1 – Replacing electrolytes. During extreme exercise, athlete will often consume drinks that contain “electrolytes,” chemical such as calcium, potassium, and sodium that play an important role in muscle contraction. Electrolytes can also be depleted in other types of [DEHYDRATION]. (36)
|
|
|
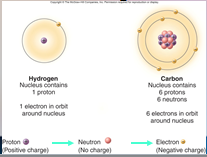
1. Figure 2.2 – Basic structure of atoms. All atoms have a nucleus consisting of protons and neutrons, except ________, the smallest atom, which has only one proton and no neutrons in its nucleus. Carbon, for example, has six protons and six neutrons in its nucleus. (36) |
![Figure 2.2 – Basic structure of atoms. All atoms have a nucleus consisting of protons and neutrons, except [HYDROGEN], the smallest atom, which has only one proton and no neutrons in its nucleus. Carbon, for example, has six protons and six neut...](https://images.cram.com/images/upload-flashcards/54/44/54/6544454_m.png)
Figure 2.2 – Basic structure of atoms. All atoms have a nucleus consisting of protons and neutrons, except [HYDROGEN], the smallest atom, which has only one proton and no neutrons in its nucleus. Carbon, for example, has six protons and six neutrons in its nucleus. (36) |
|
|
|
1. Electrons spin around the nucleus in ________ a far distance away from the nucleus. The electrons determine how atoms _____ with each other. (36)
|
Electrons spin around the nucleus in [ORBITALS] a far distance away from the nucleus. The electrons determine how atoms [REACT] with each other. (36) |
|
|
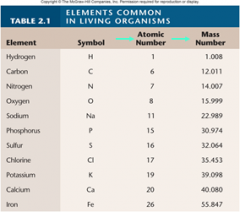
1. Table 2.1 – Elements Common in Living Organisms (36) |
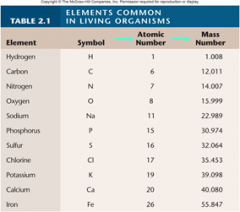
1. Table 2.1 – Elements Common in Living Organisms (36) |
|
|
|
1. _________ have very little mass (only about 1/1,840 the mass of a proton). (37)
|
[ELECTRONS] have very little mass (only about 1/1,840 the mass of a proton). (37)
|
|
|
|
1. Of all the mass contributing to your weight, the portion that is contributed by electrons is less than the mass of your eyelashes. And yet electrons determine the ________ ________ of atoms because they are the parts of atoms that come close enough to each other in nature to interact. (37)
|
Of all the mass contributing to your weight, the portion that is contributed by electrons is less than the mass of your eyelashes. And yet electrons determine the [CHEMICAL BEHAVIOR] of atoms because they are the parts of atoms that come close enough to each other in nature to interact. (37)
|
|
|
|
1. Almost all the volume of an atom is _____ _____. Protons and neutrons lie at the core of this space, whereas orbiting electrons are very far from the nucleus. (37)
|
Almost all the volume of an atom is [EMPTY SPACE]. Protons and neutrons lie at the core of this space, whereas orbiting electrons are very far from the nucleus. (37)
|
|
|
|
1. If the nucleus of an atom were the size of an apple, the orbit of the nearest ________ would be more than a mile out! (37)
|
If the nucleus of an atom were the size of an apple, the orbit of the nearest [ELECTRON] would be more than a mile out! (37)
|
|
|
|
1. Because electrons are negatively charged, they are attracted to the positively charged nucleus, but they also _____ the negative charges of each other. (37) |
Because electrons are negatively charged, they are attracted to the positively charged nucleus, but they also [REPEL] the negative charges of each other. (37) |
|
|
|
1. It takes work to keep electrons in orbit, just as it takes work to hold an apple in your hand when gravity is pulling the apple down toward the ground. The apple in your hand is said to possess ______, the ability to do work, because of its position – if you were to release it, the apple would fall. (37)
|
It takes work to keep electrons in orbit, just as it takes work to hold an apple in your hand when gravity is pulling the apple down toward the ground. The apple in your hand is said to possess [ENERGY], the ability to do work, because of its position – if you were to release it, the apple would fall. (37)
|
|
|
|
1. Electrons have energy to position, called _________ energy. (37) |
Electrons have energy to position, called [POTENTIAL] energy. (37) |
|
|
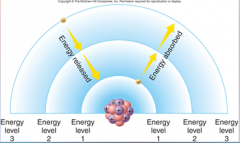
1. It takes work to oppose the attraction of the nucleus, so moving the electron farther out from the nucleus, as shown by the set of arrows on the right side of figure 2.3, requires an input of energy and results in an electron with greater potential energy. Moving an electron in toward the nucleus has the opposite effect (the set of arrows on the left side); energy is ________, and the electron has less potential energy. (37) |
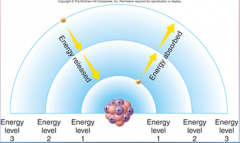
It takes work to oppose the attraction of the nucleus, so moving the electron farther out from the nucleus, as shown by the set of arrows on the right side of figure 2.3, requires an input of energy and results in an electron with greater potential energy. Moving an electron in toward the nucleus has the opposite effect (the set of arrows on the left side); energy is [RELEASED], and the electron has less potential energy. (37) |
|
|
|
1. Cells use the potential energy of atoms to drive ________ reactions. (37)
|
Cells use the potential energy of atoms to drive [CHEMICAL] reactions. (37)
|
|
|
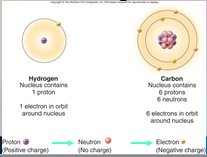
1. While the energy levels of an atom are often visualized as well-defined circular orbits around a central nucleus as was shown in figure 2.2, such a simple picture is not accurate. These energy levels, called ________ ______, often consist of complex three-dimensional shapes, and the exact location of an individual electron at any given time is impossible to specify. However, some locations are more probable than others, and it is often possible to say where an electron is most likely to be _______. (37) |
![While the energy levels of an atom are often visualized as well-defined circular orbits around a central nucleus as was shown in figure 2.2, such a simple picture is not accurate. These energy levels, called [ELECTRON SHELLS], often consist of com...](https://images.cram.com/images/upload-flashcards/54/44/90/6544490_m.png)
While the energy levels of an atom are often visualized as well-defined circular orbits around a central nucleus as was shown in figure 2.2, such a simple picture is not accurate. These energy levels, called [ELECTRON SHELLS], often consist of complex three-dimensional shapes, and the exact location of an individual electron at any given time is impossible to specify. However, some locations are more probable than others, and it is often possible to say where an electron is most likely to be [LOCATED]. (37) |
|
|
|
1. The volume of space around a nucleus where an electron is most likely to be found is called the _______ of that electron. (37)
|
The volume of space around a nucleus where an electron is most likely to be found is called the [ORBITAL] of that electron. (37) |
|
|
|
1. Each electron shell has a specific ______ of orbitals, and each orbital can hold up to ___ electrons. (37)
|
Each electron shell has a specific [NUMBER] of orbitals, and each orbital can hold up to [TWO] electrons. (37)
|
|
|
|
1. The first shell in any atom contains one s (_________) orbital. (37)
|
The first shell in any atom contains one s ([SPHERICAL]) orbital. (37)
|
|
|
|
1. Helium, shown in figure 2.4a, has one electron shell with one s orbital that corresponds to the ______ energy level. The orbital contains two electrons, shown above and below the nucleus. (37)
|
Helium, shown in figure 2.4a, has one electron shell with one s orbital that corresponds to the [LOWEST] energy level. The orbital contains two electrons, shown above and below the nucleus. (37)
|
|
|
|
1. In atoms with more than one electron shell, the second shell contains ____ p orbitals (each of them dumbbell-shaped rather than spherical) and holds up to _____ electrons. (37)
|
In atoms with more than one electron shell, the second shell contains [FOUR] p orbitals (each of them dumbbell-shaped rather than spherical) and holds up to [EIGHT] electrons. (37)
|
|
|
|
1. Nitrogen, shown in figure 2.4b, has two electron shells; the first one is completely filled with two electrons, but three of the four _ ________ in the second electron shell are not filled because nitrogen’s second shell contains only five electrons (openings in orbitals are indicated with dotted circles). (37)
|
Nitrogen, shown in figure 2.4b, has two electron shells; the first one is completely filled with two electrons, but three of the four [P ORBITALS] in the second electron shell are not filled because nitrogen’s second shell contains only five electrons (openings in orbitals are indicated with dotted circles). (37)
|
|
|
|
1. In atoms with more than two electron shells, subsequent shells also contain up to ____ orbitals and a maximum of _____ electrons. (37)
|
In atoms with more than two electron shells, subsequent shells also contain up to [FOUR] orbitals and a maximum of [EIGHT] electrons. (37)
|
|
|
|
1. Atoms with ________ electron orbitals tend to be more reactive because they lose, gain, or share electrons in order to fill their outermost electron shell. (37) |
Atoms with [UNFILLED] electron orbitals tend to be more reactive because they lose, gain, or share electrons in order to fill their outermost electron shell. (37) |
|
|
|
1. Losing, gaining, or sharing electrons is the basis for chemical reactions in which ________ _____ form between atoms. (37)
|
Losing, gaining, or sharing electrons is the basis for chemical reactions in which [CHEMICAL BONDS] form between atoms. (37)
|
|
|
|
1. Key Learning Outcome 2.1 – Atoms, the smallest particles into which a substance can be _______, are composed of electrons orbiting a _______ that contains protons and neutrons. (37) |
Key Learning Outcome 2.1 – Atoms, the smallest particles into which a substance can be [DIVIDED], are composed of electrons orbiting a [NUCLEUS] that contains protons and neutrons. (37) |
|
|
|
1. Electrons determine the chemical ________ of atoms. (37)
|
Electrons determine the chemical [BEHAVIOR] of atoms. (37)
|
|
|
|
1. Figure 2.3 – The electrons of atoms possess _________ energy. (37)
|
Figure 2.3 – The electrons of atoms possess [POTENTIAL] energy. (37)
|
|
|
|
1. Electrons that circulate rapidly around the nucleus contain energy, and depending on their ________ from the nucleus, they may contain more or less energy. (37)
|
Electrons that circulate rapidly around the nucleus contain energy, and depending on their [DISTANCE] from the nucleus, they may contain more or less energy. (37)
|
|
|
|
1. Energy level 1 is the ______ potential energy level because it is closest to the nucleus. (37)
|
Energy level 1 is the [LOWEST] potential energy level because it is closest to the nucleus. (37)
|
|
|
|
1. When an electron _______ energy, it moves from level 1 to the next higher energy level (level 2). When an electron _____ energy, it falls to a lower energy level closer to the nucleus. (37)
|
When an electron [ABSORBS] energy, it moves from level 1 to the next higher energy level (level 2). When an electron [LOSES] energy, it falls to a lower energy level closer to the nucleus. (37)
|
|
|
|
1. Figure 2.4 – Electrons in electron shells. (a) An atom of helium has two protons, two neutrons, and two electrons. The electrons fill the one orbital in its one ________ _____, the lowest energy level. (37)
|
Figure 2.4 – Electrons in electron shells. (a) An atom of helium has two protons, two neutrons, and two electrons. The electrons fill the one orbital in its one [ELECTRON SHELL], the lowest energy level. (37)
|
|
|
|
1. Sometimes an atom may ____ or ____ an electron from its outer shell. (38)
|
Sometimes an atom may [GAIN] or [LOSE] an electron from its outer shell. (38)
|
|
|
|
1. Atoms in which the number of electrons does not equal the number of protons because they have gained or lost one or more electrons are called ____. (38)
|
Atoms in which the number of electrons does not equal the number of protons because they have gained or lost one or more electrons are called [IONS]. (38)
|
|
|
|
1. All ions are electrically charged. For example, an atom of sodium (on the left in figure 2.5) becomes a positively charged ion, called a ______ (on the right), when it loses an electron, such that one proton in the nucleus is left with an unbalanced charge (11 positively charged protons and only 10 negatively charged electrons). (38)
|
All ions are electrically charged. For example, an atom of sodium (on the left in figure 2.5) becomes a positively charged ion, called a [CATION] (on the right), when it loses an electron, such that one proton in the nucleus is left with an unbalanced charge (11 positively charged protons and only 10 negatively charged electrons). (38)
|
|
|
|
1. Negatively charged ions, called ______, also form when an atom gains an electron from another atom. (38)
|
Negatively charged ions, called [ANIONS], also form when an atom gains an electron from another atom. (38)
|
|
|
|
1. The number of ________ in an atom of a particular element can vary without changing the chemical properties of the element. (38)
|
The number of [NEUTRONS] in an atom of a particular element can vary without changing the chemical properties of the element. (38)
|
|
|
|
1. Atoms that have the same number of protons but different number of neutrons are called ________. (38)
|
Atoms that have the same number of protons but different number of neutrons are called [ISOTOPES]. (38)
|
|
|
|
1. Isotopes of an atom have the same ______ number but differ in their ____ number. (38)
|
Isotopes of an atom have the same [ATOMIC] number but differ in their [MASS] number. (38)
|
|
|
|
1. ___________ isotopes are used in medicine and in dating fossils. (38)
|
[RADIOACTIVE] isotopes are used in medicine and in dating fossils. (38)
|
|
|
|
1. Isotopes can be used in many medical procedures. Short-lived isotopes, those that decay fairly rapidly and produce harmless products, are commonly used as _______ in the body. (38)
|
Isotopes can be used in many medical procedures. Short-lived isotopes, those that decay fairly rapidly and produce harmless products, are commonly used as [TRACERS] in the body. (38)
|
|
|
|
1. A ______ is a radioactive substance that is taken up and used by the body. (38)
|
A [TRACER] is a radioactive substance that is taken up and used by the body. (38)
|
|
|
|
1. Emissions from the radioactive _______ tracer are detected using special laboratory equipment and can reveal key diagnostic information about the functioning of the body. (38)
|
Emissions from the radioactive [ISOTOPE] tracer are detected using special laboratory equipment and can reveal key diagnostic information about the functioning of the body. (38)
|
|
|
|
1. PET and PET/CT (positron emission tomography/computerized tomography) imaging procedures can be used to identify a cancerous area in the body. First, a radioactive tracer is injected into the body. This tracer is taken up by all cells, but it is taken up in larger amounts in cells with higher metabolic activities, such as cancer cells. Images are then taken of the body, and areas emitting greater amounts of the tracer can be seen. For example, in the images in figure 2.7, the radioactive-emitting cancer site appears as a black area on the left and a yellow glowing area on the right. There are many other uses of radioactive isotopes in ________. (38)
|
PET and PET/CT (positron emission tomography/computerized tomography) imaging procedures can be used to identify a cancerous area in the body. First, a radioactive tracer is injected into the body. This tracer is taken up by all cells, but it is taken up in larger amounts in cells with higher metabolic activities, such as cancer cells. Images are then taken of the body, and areas emitting greater amounts of the tracer can be seen. For example, in the images in figure 2.7, the radioactive-emitting cancer site appears as a black area on the left and a yellow glowing area on the right. There are many other uses of radioactive isotopes in [MEDICINE]. (38)
|
|
|
|
1. Figure 2.6 – Isotopes of the element carbon. The three most ________ isotopes of carbon are carbon-12, carbon-13, and carbon-14. The yellow “clouds” in the diagrams represent the orbiting electrons, whose numbers are the same for all three isotopes. Protons are shown in purple, and neutrons are shown in pink. (38)
|
Figure 2.6 – Isotopes of the element carbon. The three most [ABUNDANT] isotopes of carbon are carbon-12, carbon-13, and carbon-14. The yellow “clouds” in the diagrams represent the orbiting electrons, whose numbers are the same for all three isotopes. Protons are shown in purple, and neutrons are shown in pink. (38)
|
|
|
|
1. Figure 2.7 – Using a radioactive tracer to identify cancer. In certain medical imaging procedures, the patient ingests or is injected intravenously with a radioactive tracer that is absorbed in greater amounts by cancer cells. The tracer emits radioactivity that is detected using ___ and ___/__ equipment. A cancerous area in the neck is seen in these two images, as a dark black area on the left and a bright yellow glowing area on the right. (39) |
Figure 2.7 – Using a radioactive tracer to identify cancer. In certain medical imaging procedures, the patient ingests or is injected intravenously with a radioactive tracer that is absorbed in greater amounts by cancer cells. The tracer emits radioactivity that is detected using [PET] and [PET/CT] equipment. A cancerous area in the neck is seen in these two images, as a dark black area on the left and a bright yellow glowing area on the right. (39) |
|
|
|
1. A ______ is any record of prehistoric life – generally taken to mean older than 10,000 years. (39) |
A [FOSSIL] is any record of prehistoric life – generally taken to mean older than 10,000 years. (39) |
|
|
|
1. By dating the _____ in which fossils occur, biologists can get a very good idea of how old the fossils are. (39)
|
By dating the [ROCKS] in which fossils occur, biologists can get a very good idea of how old the fossils are. (39)
|
|
|
|
1. Rocks are usually dated by measuring the degree of radioactive decay of certain radioactive ________ among rock-forming minerals. (39)
|
Rocks are usually dated by measuring the degree of radioactive decay of certain radioactive [ISOTOPES] among rock-forming minerals. (39)
|
|
|
|
1. A radioactive isotope is one whose nucleus is ________ and eventually flies apart, creating more stable atoms of another element. (39)
|
A radioactive isotope is one whose nucleus is [UNSTABLE] and eventually flies apart, creating more stable atoms of another element. (39)
|
|
|
|
1. Because the rate of decay of a radioactive element (the percent of isotopes that undergo decay in a minute) is ________, scientists can use the amount of radioactive decay to date fossils. The older the fossil, the greater the fraction of decayed radioactive isotopes. (39)
|
Because the rate of decay of a radioactive element (the percent of isotopes that undergo decay in a minute) is [CONSTANT], scientists can use the amount of radioactive decay to date fossils. The older the fossil, the greater the fraction of decayed radioactive isotopes. (39)
|
|
|
|
1. A widely employed method of dating fossils less than 50,000 years old is the carbon-14 (14C) radioisotopic dating method illustrated in figure 2.8. Most carbon atoms have a mass number of 12 (12C). However, a tiny but fixed proportion of the carbon atoms in the atmosphere consists of carbon atoms with a mass number of 14 (14C). This isotope of carbon is created by the bombardment of nitrogen-14 atoms with cosmic rays. This proportion of 14C (designated A in the figure) is captured by plants in photosynthesis and is the proportion present in the carbon molecules of the animal’s body that eats the plants, in this case a rabbit. After the plant or animal dies, it no longer accumulates carbon, and the 14C present at the time of death gradually decays over time back to nitrogen-14 (14N). The amount of 14C (A) decreases while the amount of 12C stays the same. Scientists can determine how long ago an organism died by measuring the ration of 14C to 12C in its remains or in the fossilized rock. Over time, the ratio of 14C to 12C decreases. It takes 5,730 years for half of the 14C (1/2A or A/2) present in the sample to be converted to 14N by this process. This length of time is called the half-life of the isotope. Because the half-life of an isotope is a constant that never changes, the extent of radioactive decay allows you to date a sample. Thus, a sample that had a quarter of its original proportion of 14C remaining (1/4A or A/4) would be approximately 11,460 years old (two half-lives – 5,730 years for half of the 14C to decay to a level of A/2 and another 5,730 years for the remaining 14C to decay to a level of A/4). (39)
|
A widely employed method of dating fossils less than 50,000 years old is the carbon-14 (14C) radioisotopic dating method illustrated in figure 2.8. Most carbon atoms have a mass number of 12 (12C). However, a tiny but fixed proportion of the carbon atoms in the atmosphere consists of carbon atoms with a mass number of 14 (14C). This isotope of carbon is created by the bombardment of nitrogen-14 atoms with cosmic rays. This proportion of 14C (designated A in the figure) is captured by plants in photosynthesis and is the proportion present in the carbon molecules of the animal’s body that eats the plants, in this case a rabbit. After the plant or animal dies, it no longer accumulates carbon, and the 14C present at the time of death gradually decays over time back to nitrogen-14 (14N). The amount of 14C (A) decreases while the amount of 12C stays the same. Scientists can determine how long ago an organism died by measuring the ration of 14C to 12C in its remains or in the fossilized rock. Over time, the ratio of 14C to 12C decreases. It takes 5,730 years for half of the 14C (1/2A or A/2) present in the sample to be converted to 14N by this process. This length of time is called the half-life of the isotope. Because the half-life of an isotope is a constant that never changes, the extent of radioactive decay allows you to date a sample. Thus, a sample that had a quarter of its original proportion of 14C remaining (1/4A or A/4) would be approximately 11,460 years old (two half-lives – 5,730 years for half of the 14C to decay to a level of A/2 and another 5,730 years for the remaining 14C to decay to a level of A/4). (39) |
|
|
|
1. For fossils older than 50,000 years, there is too little 14C remaining to measure precisely, and scientists instead examine the decay of potassium-40 (40K) into argon-40 (40Ar), which has a ____-____ of 1.3 billion years. (39) |
For fossils older than 50,000 years, there is too little 14C remaining to measure precisely, and scientists instead examine the decay of potassium-40 (40K) into argon-40 (40Ar), which has a [HALF-LIFE] of 1.3 billion years. (39) |
|
|
|
1. Key Learning Outcome 2.2 – When an atom gains or loses one or more electrons, it is called a(n) ___. Isotopes of an element differ in the number of ________ they contain, but all have the same chemical properties. (39)
|
Key Learning Outcome 2.2 – When an atom gains or loses one or more electrons, it is called a(n) [ION]. Isotopes of an element differ in the number of [NEUTRONS] they contain, but all have the same chemical properties. (39)
|
|
|
|
1. Figure 2.8 – Radioactive isotope dating. This diagram illustrates radioactive dating using ______-__, a short-lived isotope. (39)
|
Figure 2.8 – Radioactive isotope dating. This diagram illustrates radioactive dating using [CARBON-14], a short-lived isotope. (39)
|
|
|
|
1. A ________ is a group of atoms held together by energy. The energy acts as “glue,” ensuring that the various atoms stick to one another.(40)
|
A [MOLECULE] is a group of atoms held together by energy. The energy acts as “glue,” ensuring that the various atoms stick to one another.(40)
|
|
|
|
1. The energy or force holding two atoms together is called a ________ ____. (40)
|
The energy or force holding two atoms together is called a [CHEMICAL BOND]. (40)
|
|
|
|
1. Chemical bonds determine the ______ of the large biological molecules. (40)
|
Chemical bonds determine the [SHAPES] of the large biological molecules. (40)
|
|
|
|
1. There are three principal kinds of chemical bonds: _____ bonds, where the force is generated by the attraction of oppositely charge ions; ________ bonds, where the force results from the sharing of electrons; and ________ bonds, where the force is generated by the attraction of opposite partial electrical charges. (40)
|
There are three principal kinds of chemical bonds: [IONIC] bonds, where the force is generated by the attraction of oppositely charge ions; [COVALENT] bonds, where the force results from the sharing of electrons; and [HYDROGEN] bonds, where the force is generated by the attraction of opposite partial electrical charges. (40)
|
|
|
|
1. Another type of chemical attraction called ___ ___ _____ forces will be discussed later, but keep in mind that this type of interaction is not considered a chemical bond. (40)
|
Another type of chemical attraction called [VAN DER WAALS] forces will be discussed later, but keep in mind that this type of interaction is not considered a chemical bond. (40)
|
|
|
|
1. Chemical bonds called ionic bonds form when atoms are attracted to each other by opposite __________ charges. (40)
|
Chemical bonds called ionic bonds form when atoms are attracted to each other by opposite [ELECTRICAL] charges. (40)
|
|
|
|
1. Just as the positive pole of a magnet is attracted to the negative pole of another, so an atom can form a strong link with another atom if they have opposite electrical charges. Because an atom with an electrical charge is an ion, these bonds are called _____ _____. (40)
|
Just as the positive pole of a magnet is attracted to the negative pole of another, so an atom can form a strong link with another atom if they have opposite electrical charges. Because an atom with an electrical charge is an ion, these bonds are called [IONIC BONDS]. (40)
|
|
|
|
1. Everyday table salt is built of ionic bonds. The sodium and chlorine atoms of table salt are ____. (40)
|
Everyday table salt is built of ionic bonds. The sodium and chlorine atoms of table salt are [IONS]. (40)
|
|
|
|
1. Recall from section 2.1 that an atom is more stable when its outermost shell electron shell is filled (with ___ electrons in the innermost shell or _____ electrons in shells that are farther out from the nucleus). (40)
|
Recall from section 2.1 that an atom is more stable when its outermost shell electron shell is filled (with [TWO] electrons in the innermost shell or [EIGHT] electrons in shells that are farther out from the nucleus). (40)
|
|
|
|
1. To achieve this stability, an atom will give up or accept electrons from another ____. As a result of this electron hopping, sodium atoms in table salt are positive sodium ions and chlorine atoms are negative chloride ions. (40)
|
To achieve this stability, an atom will give up or accept electrons from another [ATOM]. As a result of this electron hopping, sodium atoms in table salt are positive sodium ions and chlorine atoms are negative chloride ions. (40)
|
|
|
|
1. Because each ion is attracted electrically to all surrounding ions of ________ charge, this causes the formation of an elaborate matrix of sodium and chloride ionic bonds – a crystal. The sodium chloride crystal reveals an organized structure of alternating sodium (yellow) and chloride (light green) ions. That is why table salt is composed of tiny crystals and is not a powder. (40)
|
Because each ion is attracted electrically to all surrounding ions of [OPPOSITE] charge, this causes the formation of an elaborate matrix of sodium and chloride ionic bonds – a crystal. The sodium chloride crystal shown above reveals an organized structure of alternating sodium (yellow) and chloride (light green) ions. That is why table salt is composed of tiny crystals and is not a powder. (40)
|
|
|
|
1. The two key properties of ionic bonds that make them form crystals are that they are strong (although not as strong as covalent bonds) and that they are not ___________. (40)
|
The two key properties of ionic bonds that make them form crystals are that they are strong (although not as strong as covalent bonds) and that they are not [DIRECTIONAL]. (40)
|
|
|
|
1. A charged atom is attracted to the __________ _____ contributed by all nearby atoms of opposite charge. Ionic bonds do not play an important part in most biological molecules because of this lack of directionality. Complex, stable shapes require the more specific associations made possible by directional bonds. (40) |
A charged atom is attracted to the [ELECTRICAL FIELD] contributed by all nearby atoms of opposite charge. Ionic bonds do not play an important part in most biological molecules because of this lack of directionality. Complex, stable shapes require the more specific associations made possible by directional bonds. (40) |
|
|
|
1. Strong chemical bonds called covalent bonds form between two atoms when they _____ electrons. Most of the atoms in your body are linked to other atoms by covalent bonds. (41)
|
Strong chemical bonds called covalent bonds form between two atoms when they [SHARE] electrons. Most of the atoms in your body are linked to other atoms by covalent bonds. (41)
|
|
|
|
1. Whey do atoms in molecules share electrons? Remember, all atoms seek to fill up their outermost shell of orbiting electrons, which in all atoms (except tiny hydrogen and helium) takes _____ electrons. (41)
|
Whey do atoms in molecules share electrons? Remember, all atoms seek to fill up their outermost shell of orbiting electrons, which in all atoms (except tiny hydrogen and helium) takes [EIGHT] electrons. (41)
|
|
|
|
1. A covalent bond is formed when electrons are shared between atoms. The atoms sharing the electrons may be of the same or different ________. Some atoms, like hydrogen (H), can form only one covalent bond, because hydrogen needs only one more electron to fill its outermost shell. (41)
|
A covalent bond is formed when electrons are shared between atoms. The atoms sharing the electrons may be of the same or different [ELEMENTS]. Some atoms, like hydrogen (H), can form only one covalent bond, because hydrogen needs only one more electron to fill its outermost shell. (41)
|
|
|
|
1. Other atoms, such as carbon (C), nitrogen (N), and oxygen (O), can form more than one covalent bond, depending on the space available in their _________ electron shells. (41)
|
Other atoms, such as carbon (C), nitrogen (N), and oxygen (O), can form more than one covalent bond, depending on the space available in their [OUTERMOST] electron shells. (41)
|
|
|
|
1. Most covalent bonds are ______ bonds, which involve the sharing of two electrons, but ______ bonds (in which four electrons are shared) are also common. ______ bonds (in which six electrons are shared) are much less frequent in nature but are found in some common compounds, like nitrogen gas (N2). (41)
|
Most covalent bonds are [SINGLE] bonds, which involve the sharing of two electrons, but [DOUBLE] bonds (in which four electrons are shared) are also common. [TRIPLE] bonds (in which six electrons are shared) are much less frequent in nature but are found in some common compounds, like nitrogen gas (N2). (41)
|
|
|
|
1. ______ is often released when covalent bonds are broken. The Hindenburg dirigible was filled with hydrogen gas when it exploded and burned in 1937; the energy of the inferno came from the breaking of H2 covalent bonds. (41)
|
[ENERGY] is often released when covalent bonds are broken. The Hindenburg dirigible was filled with hydrogen gas when it exploded and burned in 1937; the energy of the inferno came from the breaking of H2 covalent bonds. (41)
|
|
|
|
1. When a covalent bond forms between two atoms, one nucleus may be much better at __________ the shared electrons than the other, an aspect of the atom called its electronegativity. (41)
|
When a covalent bond forms between two atoms, one nucleus may be much better at [ATTRACTING] the shared electrons than the other, an aspect of the atom called its electronegativity. (41)
|
|
|
|
1. In water the shared electrons are much more strongly attracted to the oxygen atom than to the hydrogen atoms; oxygen has higher electronegativity. When this happens, shared electrons spend more time in the vicinity of the oxygen atom, which as a result becomes somewhat negative in charge; they spend less time in the vicinity of the hydrogens, and these become somewhat positive in charge. The charges are not full electrical charges like in ions but rather tiny partial charges signified by the Greek letter delta (𝛿). What you end up with is a sort of molecular magnet, with positive and negative ends, or “poles.” Molecules like this are said to be polar molecules, and the bonds between the atoms are called polar covalent bonds. (41)
|
In water the shared electrons are much more strongly attracted to the oxygen atom than to the hydrogen atoms; oxygen has higher electronegativity. When this happens, shared electrons spend more time in the vicinity of the oxygen atom, which as a result becomes somewhat negative in charge; they spend less time in the vicinity of the hydrogens, and these become somewhat positive in charge. The charges are not full electrical charges like in ions but rather tiny partial charges signified by the Greek letter delta (𝛿). What you end up with is a sort of molecular magnet, with positive and negative ends, or “poles.” Molecules like this are said to be polar molecules, and the bonds between the atoms are called polar covalent bonds. (41) |
|
|
|
1. Molecules that don’t exhibit a large difference in ___________________ of its atoms, like the carbon-hydrogen bonds of methane, are called nonpolar molecules and contain nonpolar covalent bonds. (41)
|
Molecules that don’t exhibit a large difference in [ELECTRONEGATIVITIES] of its atoms, like the carbon-hydrogen bonds of methane, are called nonpolar molecules and contain nonpolar covalent bonds. (41)
|
|
|
|
1. The two key properties of covalent bonds that make them ideal for their molecule-building role in living systems are that (1) they are ______, involving the sharing of lots of energy; and (2) they are very ___________ – bonds form between two specific atoms, rather than a generalized attraction of one atom for its neighbors. (41)
|
The two key properties of covalent bonds that make them ideal for their molecule-building role in living systems are that (1) they are [STRONG], involving the sharing of lots of energy; and (2) they are very [DIRECTIONAL] – bonds form between two specific atoms, rather than a generalized attraction of one atom for its neighbors. (41)
|
|
|
|
1. Polar molecules like water are attracted to one another, a special type of weak chemical bond called a hydrogen bond. Hydrogen bonds occur when the ________ end of one polar molecule is attracted to the ________ end of another, like two magnets drawn to each other. (42)
|
Polar molecules like water are attracted to one another, a special type of weak chemical bond called a hydrogen bond. Hydrogen bonds occur when the [POSITIVE] end of one polar molecule is attracted to the [NEGATIVE] end of another, like two magnets drawn to each other. (42)
|
|
|
|
1. In a hydrogen bond, an _______________ hydrogen from one polar molecule is attracted to an _______________ atom, often oxygen (O) or nitrogen (N), from another polar molecule. (42)
|
In a hydrogen bond, an [ELECTROPOSITIVE] hydrogen from one polar molecule is attracted to an [ELECTRONEGATIVE] atom, often oxygen (O) or nitrogen (N), from another polar molecule. (42)
|
|
|
|
1. Because the oxygen atoms in water molecules are more electronegative than the hydrogen atoms, water molecules are _____. Water molecules form ______ hydrogen bonds with each other, giving liquid water many unique properties. (42)
|
Because the oxygen atoms in water molecules are more electronegative than the hydrogen atoms, water molecules are [POLAR]. Water molecules form [STRONG] hydrogen bonds with each other, giving liquid water many unique properties. (42)
|
|
|
|
1. Each oxygen has a partial negative charge (𝛿-), and each hydrogen has a partial positive charge (𝛿+). ________ _____ (shown as dashed lines) form between the positive end of one polar molecule and the negative end of another polar molecule. This attraction of partial charges attracts water molecules to one another. (42)
|
Each oxygen has a partial negative charge (𝛿-), and each hydrogen has a partial positive charge (𝛿+). [HYDROGEN BONDS] (shown as dashed lines) form between the positive end of one polar molecule and the negative end of another polar molecule. This attraction of partial charges attracts water molecules to one another. (42)
|
|
|
|
1. Two key properties of hydrogen bonds cause them to play an important role in the molecules found in organisms. First, they are ____ and so are not effective over long distances like more powerful covalent and ionic bonds. Hydrogen bonds are too weak to actually form stable molecules by themselves. Instead, they act like Velcro, forming a tight bond by the additive effects of many weak interactions. Second, hydrogen bonds are highly ___________. (42) |
Two key properties of hydrogen bonds cause them to play an important role in the molecules found in organisms. First, they are [WEAK] and so are not effective over long distances like more powerful covalent and ionic bonds. Hydrogen bonds are too weak to actually form stable molecules by themselves. Instead, they act like Velcro, forming a tight bond by the additive effects of many weak interactions. Second, hydrogen bonds are highly [DIRECTIONAL]. (42) |
|
|
|
1. Another important kind of weak chemical attraction is a ___-___________ attractive force called van der Waals forces (or van der Waals interactions). These chemical forces come into play only when two atoms are very _____ to one another. The attraction is very weak and disappears if the atoms move even a little apart. It becomes significant when numerous atoms in one molecule simultaneously come close to numerous atoms of another molecule – that is, when the shapes of the molecules match precisely. For example, this interaction is important when antibodies in your blood recognize the shape of an invading virus as foreign. (42) |
Another important kind of weak chemical attraction is a [NON-DIRECTIONAL] attractive force called van der Waals forces (or van der Waals interactions). These chemical forces come into play only when two atoms are very [CLOSE] to one another. The attraction is very weak and disappears if the atoms move even a little apart. It becomes significant when numerous atoms in one molecule simultaneously come close to numerous atoms of another molecule – that is, when the shapes of the molecules match precisely. For example, this interaction is important when antibodies in your blood recognize the shape of an invading virus as foreign. (42) |
|
|
|
1. Key Learning Outcome 2.3 – Molecules are atoms ______ together by chemical bonds. Ionic bonds, covalent bonds, and hydrogen bonds are the three _________ types of bonds, and van der Waals forces are weaker interactions. (42)
|
Key Learning Outcome 2.3 – Molecules are atoms [LINKED] together by chemical bonds. Ionic bonds, covalent bonds, and hydrogen bonds are the three [PRINCIPAL] types of bonds, and van der Waals forces are weaker interactions. (42)
|
|
|
|
1. Water has a simple ______ structure, an oxygen atom linked to two hydrogen atoms by single ________ bonds. The chemical formula for water is thus H20. (43)
|
Water has a simple [ATOMIC] structure, an oxygen atom linked to two hydrogen atoms by single [COVALENT] bonds. The chemical formula for water is thus H20. (43)
|
|
|
|
1. It is because the oxygen atom attracts the shared electrons more strongly than the hydrogen atoms that water is a _____ molecule and so can form hydrogen bonds. Water’s ability to form hydrogen bonds is responsible for much of the organization of living chemistry, from membrane structure to how proteins fold. (43)
|
It is because the oxygen atom attracts the shared electrons more strongly than the hydrogen atoms that water is a [POLAR] molecule and so can form hydrogen bonds. Water’s ability to form hydrogen bonds is responsible for much of the organization of living chemistry, from membrane structure to how proteins fold. (43)
|
|
|
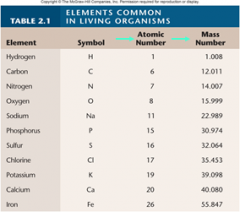
1. The ____ hydrogen bonds that form between a hydrogen atom of one water molecule and the oxygen atom of another produce a lattice of hydrogen bonds within liquid water. Each of these bonds is individually very weak and short-lived – a single bond lasts only 1/100,000,000,000 of a second. However, like the grains of sand on a beach, the cumulative effect of large numbers of these bonds is enormous and is responsible for many of the important physical properties of water (table 2.2). (43) |
![The [WEAK] hydrogen bonds that form between a hydrogen atom of one water molecule and the oxygen atom of another produce a lattice of hydrogen bonds within liquid water. Each of these bonds is individually very weak and short-lived – a single bo...](https://images.cram.com/images/upload-flashcards/54/46/01/6544601_m.png)
The [WEAK] hydrogen bonds that form between a hydrogen atom of one water molecule and the oxygen atom of another produce a lattice of hydrogen bonds within liquid water. Each of these bonds is individually very weak and short-lived – a single bond lasts only 1/100,000,000,000 of a second. However, like the grains of sand on a beach, the cumulative effect of large numbers of these bonds is enormous and is responsible for many of the important physical properties of water (table 2.2). (43) |
|
|
|
1. The temperature of any substance is a measure of how rapidly its individual _________ are moving. (43)
|
The temperature of any substance is a measure of how rapidly its individual [MOLECULES] are moving. (43) |
|
|
|
1. Because of the many hydrogen bonds that water molecules form with one another, a large input of _______ ______ is required to disrupt the organization of liquid water and raise its temperature. Because of this, water heats up more slowly than almost any other compound and holds its temperature ______. That is a major reason why your body is able to maintain a relatively constant internal temperature. (43) |
Because of the many hydrogen bonds that water molecules form with one another, a large input of [THERMAL ENERGY] is required to disrupt the organization of liquid water and raise its temperature. Because of this, water heats up more slowly than almost any other compound and holds its temperature [LONGER]. That is a major reason why your body is able to maintain a relatively constant internal temperature. (43) |
|
|
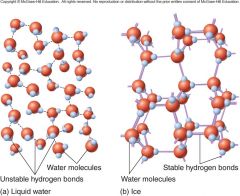
1. If the temperature is low enough, very few hydrogen bonds _____ in water. Instead, the lattice of these bonds assumes a crystal-like structure, forming a solid we call ice. Interestingly, ice is less dense than water – that is why icebergs and ice cubes float. Why is ice less dense? This is best understood by comparing the molecular structures of water and ice that you see in figure 2.9. At temperature above freezing (0˚C or 32˚F), water molecules in figure 2.9a move around each other with hydrogen bonds breaking and forming. As temperatures drop, the movement of water molecules decreases, allowing hydrogen bonds to _________, holding individual molecules farther apart, as in figure 2.9b, making the ice structure less dense. (43) |
![If the temperature is low enough, very few hydrogen bonds [BREAK] in water. Instead, the lattice of these bonds assumes a crystal-like structure, forming a solid we call ice. Interestingly, ice is less dense than water – that is why icebergs and...](https://images.cram.com/images/upload-flashcards/54/46/10/6544610_m.jpg)
If the temperature is low enough, very few hydrogen bonds [BREAK] in water. Instead, the lattice of these bonds assumes a crystal-like structure, forming a solid we call ice. Interestingly, ice is less dense than water – that is why icebergs and ice cubes float. Why is ice less dense? This is best understood by comparing the molecular structures of water and ice that you see in figure 2.9. At temperature above freezing (0˚C or 32˚F), water molecules in figure 2.9a move around each other with hydrogen bonds breaking and forming. As temperatures drop, the movement of water molecules decreases, allowing hydrogen bonds to [STABILIZE], holding individual molecules farther apart, as in figure 2.9b, making the ice structure less dense. (43) |
|
|
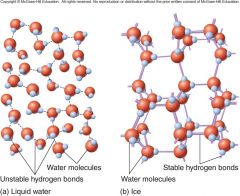
1. Figure 2.9 – Ice Formation. When water (a) cools below 0˚C, it forms a regular crystal structure (b) that floats. The individual water molecules are ______ apart and held in position by hydrogen bonds. (43) |
![Figure 2.9 – Ice Formation. When water (a) cools below 0˚C, it forms a regular crystal structure (b) that floats. The individual water molecules are [SPACED] apart and held in position by hydrogen bonds. (43)](https://images.cram.com/images/upload-flashcards/54/46/16/6544616_m.jpg)
Figure 2.9 – Ice Formation. When water (a) cools below 0˚C, it forms a regular crystal structure (b) that floats. The individual water molecules are [SPACED] apart and held in position by hydrogen bonds. (43) |
|
|
|
1. If the temperature is high enough, many hydrogen bonds _____ in water, with the result that the liquid is changed into vapor. A considerable amount of heat energy is required to do this – every gram of water that evaporates from your skin removes 2,452 joules of heat from your body, which is equal to the energy released by ________ the temperature of 586 grams of water 1˚C. That is why sweating cools you off; as the sweat evaporates (vaporizes) it takes energy with it, in the form of heat, cooling the body. (43)
|
If the temperature is high enough, many hydrogen bonds [BREAK] in water, with the result that the liquid is changed into vapor. A considerable amount of heat energy is required to do this – every gram of water that evaporates from your skin removes 2,452 joules of heat from your body, which is equal to the energy released by [LOWERING] the temperature of 586 grams of water 1˚C. That is why sweating cools you off; as the sweat evaporates (vaporizes) it takes energy with it, in the form of heat, cooling the body. (43) |
|
|
|
1. Because water molecules are very polar, they are attracted to other polar molecules – ________ bonds bind polar molecules to each other. (44)
|
Because water molecules are very polar, they are attracted to other polar molecules – [HYDROGEN] bonds bind polar molecules to each other. (44)
|
|
|
|
1. When the other polar molecule is another water molecule, the attraction is called ________. (44)
|
When the other polar molecule is another water molecule, the attraction is called [COHESION]. (44)
|
|
|
|
1. The surface tension of water is created by cohesion. Surface tension is the _____ that causes water to bead, like on the spider web in figure 2.10, or supports the weight of the water strider. (44)
|
The surface tension of water is created by cohesion. Surface tension is the [FORCE] that causes water to bead, like on the spider web in figure 2.10, or supports the weight of the water strider. (44) |
|
|
|
1. When the other polar molecule is a different substance, the attraction is called ________. (44)
|
When the other polar molecule is a different substance, the attraction is called [ADHESION]. (44)
|
|
|
|
1. Capillary action – such as water moving up into a paper towel – is created by adhesion. Water clings to any substance, such as paper fibers, with which it can form ________ bonds. Adhesion is why things get “wet” when they are dipped in water and why waxy substances do not – they are composed of ________ molecules that don’t form hydrogen bonds with water molecules. (44)
|
Capillary action – such as water moving up into a paper towel – is created by adhesion. Water clings to any substance, such as paper fibers, with which it can form [HYDROGEN] bonds. Adhesion is why things get “wet” when they are dipped in water and why waxy substances do not – they are composed of [NONPOLAR] molecules that don’t form hydrogen bonds with water molecules. (44) |
|
|
|
1. Figure 2.10 – Cohesion. (a) Cohesion allows water molecules to _____ together and form droplets. (b) _______ _______ is a property derived from cohesion – that is, water has a “strong” surface due to the force of its hydrogen bonds. Some insects, such as this water strider, literally walk on water. (44)
|
Figure 2.10 – Cohesion. (a) Cohesion allows water molecules to [STICK] together and form droplets. (b) [SURFACE TENSION] is a property derived from cohesion – that is, water has a “strong” surface due to the force of its hydrogen bonds. Some insects, such as this water strider, literally walk on water. (44)
|
|
|
|
1. Water molecules in solution always tend to form the _______ number of hydrogen bonds possible. (44)
|
Water molecules in solution always tend to form the [MAXIMUM] number of hydrogen bonds possible. (44)
|
|
|
|
1. _____ molecules form hydrogen bonds and are attracted to water molecules. (44)
|
[POLAR] molecules form hydrogen bonds and are attracted to water molecules. (44)
|
|
|
|
1. Polar molecules are called hydrophilic (from the Greek hydros, water, and philic, loving), or water-loving, molecules. Water molecules gather closely around any molecule that exhibits an electrical charge, whether a full charge (___) or partial charge (_____ molecule). (44)
|
Polar molecules are called hydrophilic (from the Greek hydros, water, and philic, loving), or water-loving, molecules. Water molecules gather closely around any molecule that exhibits an electrical charge, whether a full charge (ION) or partial charge ([POLAR] molecule). (44)
|
|
|
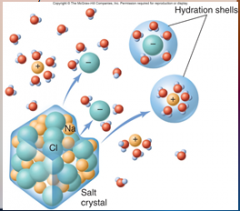
1. When a salt crystal dissolves in water as you see happening in figure 2.11, what really happens is that individual ____ break off from the crystal and become surrounded by water molecules. The blue hydrogen atoms of water are attracted to the negative charge of the chloride ions, and the red oxygen atoms are attracted to the positive charge of the sodium ions. Water molecules orient around each ion like a swarm of bees attracted to honey, and this shell of water molecules, called a _________ _____, prevents the ion from reassociating with the crystal. Similar shells of water form around all polar molecules, and polar molecules that dissolve in water in this way are said to be _______ in water. (44) |
![When a salt crystal dissolves in water as you see happening in figure 2.11, what really happens is that individual [IONS] break off from the crystal and become surrounded by water molecules. The blue hydrogen atoms of water are attracted to the ne...](https://images.cram.com/images/upload-flashcards/54/46/58/6544658_m.png)
When a salt crystal dissolves in water as you see happening in figure 2.11, what really happens is that individual [IONS] break off from the crystal and become surrounded by water molecules. The blue hydrogen atoms of water are attracted to the negative charge of the chloride ions, and the red oxygen atoms are attracted to the positive charge of the sodium ions. Water molecules orient around each ion like a swarm of bees attracted to honey, and this shell of water molecules, called a [HYDRATION SHELL], prevents the ion from reassociating with the crystal. Similar shells of water form around all polar molecules, and polar molecules that dissolve in water in this way are said to be [SOLUBLE] in water. (44) |
|
|
|
1. Nonpolar molecules like oil do not form hydrogen bonds and are not _____-_______. When nonpolar molecules are placed in water, the water molecules shy away, instead forming hydrogen bonds with other water molecules. The nonpolar molecules are forced into association with one another, crowded together to minimize their disruption of the hydrogen bonding of water. (44)
|
Nonpolar molecules like oil do not form hydrogen bonds and are not [WATER-SOLUBLE]. When nonpolar molecules are placed in water, the water molecules shy away, instead forming hydrogen bonds with other water molecules. The nonpolar molecules are forced into association with one another, crowded together to minimize their disruption of the hydrogen bonding of water. (44) |
|
|
|
1. It seems almost as if the ________ compounds shrink from contact with water, and for this reason they are called hydrophobic (from the Greek hydros, water, and phobos, fearing). Many biological structures are shaped by such hydrophobic forces. (44)
|
It seems almost as if the [NONPOLAR] compounds shrink from contact with water, and for this reason they are called hydrophobic (from the Greek hydros, water, and phobos, fearing). Many biological structures are shaped by such hydrophobic forces. (44)
|
|
|
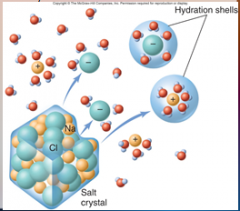
1. Figure 2.11 – How salt dissolves in water. Salt is _______ in water because the partial charges on water molecules are attracted to the charged sodium and chloride ions. The water molecules surround the ions, forming what are called _________ ______. When all of the ions have been separated from the crystal, the salt is aid to be dissolved. (44) |
![Figure 2.11 – How salt dissolves in water. Salt is [SOLUBLE] in water because the partial charges on water molecules are attracted to the charged sodium and chloride ions. The water molecules surround the ions, forming what are called [HYDRATION...](https://images.cram.com/images/upload-flashcards/54/46/67/6544667_m.png)
Figure 2.11 – How salt dissolves in water. Salt is [SOLUBLE] in water because the partial charges on water molecules are attracted to the charged sodium and chloride ions. The water molecules surround the ions, forming what are called [HYDRATION SHELLS]. When all of the ions have been separated from the crystal, the salt is aid to be dissolved. (44) |
|
|
|
1. Key Learning Outcome 2.4 – Water molecules form a network of hydrogen bonds in liquid and dissolve other _____ _________. Many of the key properties of water arise because it takes considerable ______ to break liquid water’s many hydrogen bonds. (44)
|
Key Learning Outcome 2.4 – Water molecules form a network of hydrogen bonds in liquid and dissolve other [POLAR MOLECULES]. Many of the key properties of water arise because it takes considerable [ENERGY] to break liquid water’s many hydrogen bonds. (44)
|
|
|
|
1. The ________ _____ within a water molecule sometimes break spontaneously. When it happens, a proton (hydrogen atom nucleus) dissociates from the molecule. Because the dissociated proton lacks the negatively charged electron it was sharing in the covalent bond with oxygen, its own positive charge is no longer counterbalanced, and it becomes a positively charged ion, hydrogen ion (H+). The rest of the dissociated water molecule, which has retained the shared electron from the covalent bond, is negatively charged and forms a hydroxide ion (OH-). This process of spontaneous ion formation is called __________. It can be represented by a simple chemical equation in which the chemical formulas for water and the two ions are written down with an arrow showing the direction of the dissociation: H20 (water) |
The [COVALENT BONDS ]within a water molecule sometimes break spontaneously. When it happens, a proton (hydrogen atom nucleus) dissociates from the molecule. Because the dissociated proton lacks the negatively charged electron it was sharing in the covalent bond with oxygen, its own positive charge is no longer counterbalanced, and it becomes a positively charged ion, hydrogen ion (H+). The rest of the dissociated water molecule, which has retained the shared electron from the covalent bond, is negatively charged and forms a hydroxide ion (OH-). This process of spontaneous ion formation is called [IONIZATION]. It can be represented by a simple chemical equation in which the chemical formulas for water and the two ions are written down with an arrow showing the direction of the dissociation: H20 (water) OH- (hydroxide ion) + H+ (hydrogen ion) (45) |
The [COVALENT BONDS ]within a water molecule sometimes break spontaneously. When it happens, a proton (hydrogen atom nucleus) dissociates from the molecule. Because the dissociated proton lacks the negatively charged electron it was sharing in the covalent bond with oxygen, its own positive charge is no longer counterbalanced, and it becomes a positively charged ion, hydrogen ion (H+). The rest of the dissociated water molecule, which has retained the shared electron from the covalent bond, is negatively charged and forms a hydroxide ion (OH-). This process of spontaneous ion formation is called [IONIZATION]. It can be represented by a simple chemical equation in which the chemical formulas for water and the two ions are written down with an arrow showing the direction of the dissociation: H20 (water)
|
|
|
1. Because covalent bonds are ______, spontaneous ionization is not common, In a liter of water, only roughly 1 molecule out of each 550 million is ionized at any instant in time, corresponding to 1/10,000,000 (that is, 10-7) of a mole of hydrogen ions. (A mole is a measurement of weight. One mole of any object is the weight of 6,022x1023 units of that object.) The _____________ of H+ in water can be written more easily by simply counting the number of decimal place after the digit “1” in the denominator: [H+] = 1/10,000,000 (45)
|
Because covalent bonds are [STRONG], spontaneous ionization is not common, In a liter of water, only roughly 1 molecule out of each 550 million is ionized at any instant in time, corresponding to 1/10,000,000 (that is, 10-7) of a mole of hydrogen ions. (A mole is a measurement of weight. One mole of any object is the weight of 6,022x1023 units of that object.) The [CONCENTRATION] of H+ in water can be written more easily by simply counting the number of decimal place after the digit “1” in the denominator: [H+] = 1/10,000,000 (45) |
|
|
![1. A more convenient way to express the hydrogen ion concentration of a solution is to use the __ scale (figure 2.12). This scale defines __ as the negative logarithm of the hydrogen ion concentration in the solution: pH = -log [H+] (45)](https://images.cram.com/images/upload-flashcards/54/47/27/6544727_m.jpg)
1. A more convenient way to express the hydrogen ion concentration of a solution is to use the __ scale (figure 2.12). This scale defines __ as the negative logarithm of the hydrogen ion concentration in the solution: pH = -log [H+] (45) |
![A more convenient way to express the hydrogen ion concentration of a solution is to use the [pH] scale (figure 2.12). This scale defines [pH] as the negative logarithm of the hydrogen ion concentration in the solution: pH = -log [H+] (45)](https://images.cram.com/images/upload-flashcards/54/47/24/6544724_m.jpg)
A more convenient way to express the hydrogen ion concentration of a solution is to use the [pH] scale (figure 2.12). This scale defines [pH] as the negative logarithm of the hydrogen ion concentration in the solution: pH = -log [H+] (45) |
|
|
|
1. Since the logarithm of the hydrogen ion concentration is simply the exponent of the molar concentration of H+, the pH equals the exponent time -1. Thus, pure water, with an [H+] of 10-7 mole/liter, has a pH of 7. Recall that for every hydrogen ion formed when water ___________, a hydroxide ion is also formed, meaning that the dissociation of water produces H+ and OH- in _____ amounts. Therefore, a pH value of 7 indicates neutrality – a balance between H+ and OH- - on the pH scale. (45)
|
Since the logarithm of the hydrogen ion concentration is simply the exponent of the molar concentration of H+, the pH equals the exponent time -1. Thus, pure water, with an [H+] of 10-7 mole/liter, has a pH of 7. Recall that for every hydrogen ion formed when water [DISSOCIATES], a hydroxide ion is also formed, meaning that the dissociation of water produces H+ and OH- in [EQUAL] amounts. Therefore, a pH value of 7 indicates neutrality – a balance between H+ and OH- - on the pH scale. (45) |
|
|
|
1. Note that the pH scale is ___________, which means that a difference of 1 on the pH scale represents a 10-fold change in hydrogen ion concentration. This means that a solution with a pH of 4 has 10 times the concentration of H+ present in one with a pH of 5. (45)
|
Note that the pH scale is [LOGARITHMIC], which means that a difference of 1 on the pH scale represents a 10-fold change in hydrogen ion concentration. This means that a solution with a pH of 4 has 10 times the concentration of H+ present in one with a pH of 5. (45)
|
|
|
|
1. Any substance that dissociates in water to increase the concentration of H+ is called a(n) ____. (45)
|
Any substance that dissociates in water to increase the concentration of H+ is called a(n) [ACID]. (45)
|
|
|
|
1. ______ solutions have pH values below 7. (45)
|
[ACIDIC] solutions have pH values below 7. (45)
|
|
|
|
1. The ________ an acid is, the more H+ it produces and the lower its pH. The pH of champagne, which bubbles because of the carbonic acid dissolved in it, is about 3. (45)
|
The [STRONGER] an acid is, the more H+ it produces and the lower its pH. The pH of champagne, which bubbles because of the carbonic acid dissolved in it, is about 3. (45)
|
|
|
|
1. A substance that combines with H+ when dissolved in water is called a ____. (45)
|
A substance that combines with H+ when dissolved in water is called a [BASE]. (45)
|
|
|
|
1. By combining with H+, a base lowers the H+ concentration in the solution. Basic (or ________) solutions, therefore, have pH values above 7. (45)
|
By combining with H+, a base lowers the H+ concentration in the solution. Basic (or [ALKALINE]) solutions, therefore, have pH values above 7. (45)
|
|
|
|
1. Very strong bases, such as sodium hydroxide (NaOH), have pH values of __ or more. (45)
|
Very strong bases, such as sodium hydroxide (NaOH), have pH values of [12] or more. (45)
|
|
|
|
1. The pH inside almost all living cells, and in the fluid surrounding cells in multicellular organisms, is fairly close to _. (47)
|
The pH inside almost all living cells, and in the fluid surrounding cells in multicellular organisms, is fairly close to [7]. (47)
|
|
|
|
1. The many ________ that govern metabolism are all extremely sensitive to pH, and slight alterations in pH can cause the molecules to take on different shapes that disrupt their activities. For this reason, it is important that a cell maintain a ________ pH level. (47)
|
The many [PROTEINS] that govern metabolism are all extremely sensitive to pH, and slight alterations in pH can cause the molecules to take on different shapes that disrupt their activities. For this reason, it is important that a cell maintain a [CONSTANT] pH level. (47)
|
|
|
|
1. The pH of your blood, for example, is 7.4, and you would survive only a few minutes if it were to fall to _._ or rise to _._. (47)
|
The pH of your blood, for example, is 7.4, and you would survive only a few minutes if it were to fall to [7.0] or rise to [7.8]. (47)
|
|
|
|
1. The chemical reactions of life constantly produce _____ and _____ within cells. Many animals eat substances that are acidic or basic; Coca-Cola, for example, is acidic, and egg white is basic. (47)
|
The chemical reactions of life constantly produce [ACIDS] and [BASES] within cells. Many animals eat substances that are acidic or basic; Coca-Cola, for example, is acidic, and egg white is basic. (47)
|
|
|
|
1. What keeps an organism’s pH constant? Cells contain chemical substances called _______ that minimize changes in concentrations of H+ and OH-. (47)
|
What keeps an organism’s pH constant? Cells contain chemical substances called [BUFFERS] that minimize changes in concentrations of H+ and OH-. (47)
|
|
|
|
1. A buffer is a substance that takes up or releases hydrogen ions into solution as the hydrogen ion concentration of the solution changes. Hydrogen ions are _______ to the solution when their concentration falls and _____ from the solution when their concentration rises. (47)
|
A buffer is a substance that takes up or releases hydrogen ions into solution as the hydrogen ion concentration of the solution changes. Hydrogen ions are [DONATED] to the solution when their concentration falls and [TAKEN] from the solution when their concentration rises. (47)
|
|
|
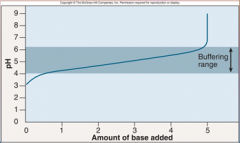
1. The graph in figure 2.13 shows how buffers work. The blue line indicates changes in pH. As a base is added to the solution, the H+ concentration _____ and the pH should ____ sharply, but by contributing H+ to the solution, the buffer works to keep the pH within a range, called the buffering range (the darker blue bar). Only when the buffering capacity is exceeded does the pH begin to rise. What sort of substance will act in this way? Within organisms, most buffers consist of pairs of substances, one an acid and the other a base. (47) |
![The graph in figure 2.13 shows how buffers work. The blue line indicates changes in pH. As a base is added to the solution, the H+ concentration [FALLS] and the pH should [RISE] sharply, but by contributing H+ to the solution, the buffer works to ...](https://images.cram.com/images/upload-flashcards/54/47/42/6544742_m.png)
The graph in figure 2.13 shows how buffers work. The blue line indicates changes in pH. As a base is added to the solution, the H+ concentration [FALLS] and the pH should [RISE] sharply, but by contributing H+ to the solution, the buffer works to keep the pH within a range, called the buffering range (the darker blue bar). Only when the buffering capacity is exceeded does the pH begin to rise. What sort of substance will act in this way? Within organisms, most buffers consist of pairs of substances, one an acid and the other a base. (47) |
|
|
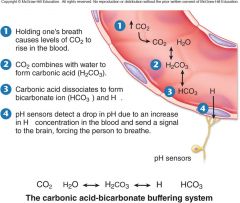
1. The key buffer in human blood is an ____-____ pair consisting of carbonic acid (acid) and bicarbonate (base). These two substances interact in a pair of reversible reactions. First, carbon dioxide (CO2) and H2O join to form carbonic acid (H2CO3) (step 2 in figure 2.14), which in a second reaction dissociates to yield bicarbonate ion (HCO3-) and H+ (step 3). If some acid or other substance adds H+ to the blood, the HCO3- acts as a base and removes the excess H+ by forming H2CO3. Similarly, if a basic substance removes H+ from the blood, H2CO3 dissociates, releasing more H+ into the blood. The forward and reverse reactions that interconvert H2CO3 and HCO3- thus _________ the blood’s pH. (47) |
![The key buffer in human blood is an [ACID-BASE] pair consisting of carbonic acid (acid) and bicarbonate (base). These two substances interact in a pair of reversible reactions. First, carbon dioxide (CO2) and H2O join to form carbonic acid (H2CO3)...](https://images.cram.com/images/upload-flashcards/54/47/60/6544760_m.jpg)
The key buffer in human blood is an [ACID-BASE] pair consisting of carbonic acid (acid) and bicarbonate (base). These two substances interact in a pair of reversible reactions. First, carbon dioxide (CO2) and H2O join to form carbonic acid (H2CO3) (step 2 in figure 2.14), which in a second reaction dissociates to yield bicarbonate ion (HCO3-) and H+ (step 3). If some acid or other substance adds H+ to the blood, the HCO3- acts as a base and removes the excess H+ by forming H2CO3. Similarly, if a basic substance removes H+ from the blood, H2CO3 dissociates, releasing more H+ into the blood. The forward and reverse reactions that interconvert H2CO3 and HCO3- thus [STABILIZE] the blood’s pH. (47) |
|
|
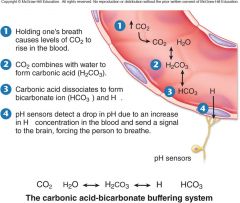
1. When you breathe in, your body takes up oxygen from the air and when you breathe out, your body releases carbon dioxide. When you hold your breath, CO2 releases carbon dioxide. When you hold your breath, CO2 accumulates in your blood and drives the chemical reactions in figure 2.14, producing carbonic acid. Can you hold your breath indefinitely? No, but not for the reason you might think. It is not lack of oxygen that forces you to breathe but too much carbon dioxide. If you try to hold your breath for very long, CO2 accumulates in the blood, as shown in step 1 in figure 2.14, triggering the formation of carbonic acid (step 2) that dissociates into bicarbonate ion and H+ (step 3). This increase in H+ causes the blood to become more acidic. If the pH in the blood drops too low, pH sensors that are located in some of the major blood vessels of the body detect the change (step 4) and send signals to the brain. These signals, along with other sensory processes, stimulate the area of the brain that controls respiration, causing it to increase the rate of breathing. Hyperventilating, breathing very quickly, has the opposite effect, lowering the levels of CO2 in the blood. That is why you are told to breathe into a paper bag when you are hyperventilating, to increase your intake of CO2. (47) |
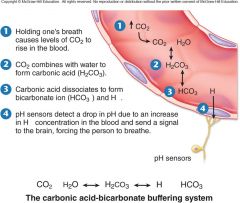
When you breathe in, your body takes up oxygen from the air and when you breathe out, your body releases carbon dioxide. When you hold your breath, CO2 releases carbon dioxide. When you hold your breath, CO2 accumulates in your blood and drives the chemical reactions in figure 2.14, producing carbonic acid. Can you hold your breath indefinitely? No, but not for the reason you might think. It is not lack of oxygen that forces you to breathe but too much carbon dioxide. If you try to hold your breath for very long, CO2 accumulates in the blood, as shown in step 1 in figure 2.14, triggering the formation of carbonic acid (step 2) that dissociates into bicarbonate ion and H+ (step 3). This increase in H+ causes the blood to become more acidic. If the pH in the blood drops too low, pH sensors that are located in some of the major blood vessels of the body detect the change (step 4) and send signals to the brain. These signals, along with other sensory processes, stimulate the area of the brain that controls respiration, causing it to increase the rate of breathing. Hyperventilating, breathing very quickly, has the opposite effect, lowering the levels of CO2 in the blood. That is why you are told to breathe into a paper bag when you are hyperventilating, to increase your intake of CO2. (47) |
|
|
|
1. Key Learning Outcome 2.5 – A tiny fraction of water molecules spontaneously ionize at any moment, forming H+ and OH-. The pH of a solution is a measure of its H+ concentration. Low pH of a solution is a measure of its H+ concentration. Low pH values indicate high H+ concentration (______ solutions), and high pH values indicate low H+ concentrations (_____ solutions). (47)
|
Key Learning Outcome 2.5 – A tiny fraction of water molecules spontaneously ionize at any moment, forming H+ and OH-. The pH of a solution is a measure of its H+ concentration. Low pH of a solution is a measure of its H+ concentration. Low pH values indicate high H+ concentration ([ACIDIC] solutions), and high pH values indicate low H+ concentrations ([BASIC] solutions). (47)
|
|
|
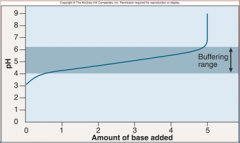
1. Figure 2.13 – Buffers minimize changes in pH. Adding a ____ to a solution neutralizes some of the acid present and so raises the pH. Thus, as the curve moves to the right, reflecting more and more base, it also rises to higher pH values. What a buffer does is to make the curve rise or fall very slowly over a portion of the pH scale, called the “_________ _____” of that buffer. (47) |
![Figure 2.13 – Buffers minimize changes in pH. Adding a [BASE] to a solution neutralizes some of the acid present and so raises the pH. Thus, as the curve moves to the right, reflecting more and more base, it also rises to higher pH values. What ...](https://images.cram.com/images/upload-flashcards/54/47/84/6544784_m.png)
Figure 2.13 – Buffers minimize changes in pH. Adding a [BASE] to a solution neutralizes some of the acid present and so raises the pH. Thus, as the curve moves to the right, reflecting more and more base, it also rises to higher pH values. What a buffer does is to make the curve rise or fall very slowly over a portion of the pH scale, called the “[BUFFERING RANGE]” of that buffer. (47) |
|
|
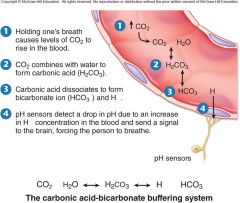
1. Figure 2.14 – Holding your breath. CO2 accumulates in the blood when a person holds his or her breath. CO2 combines with water, forming carbonic acid. Carbonic acid dissociates into bicarbonate and H+, which acts to lower the pH. The drop in pH is detected by sensors that stimulate the brain, causing the person to _______. (47) |
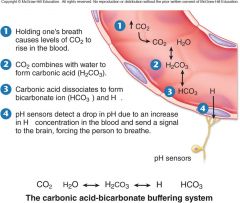
Figure 2.14 – Holding your breath. CO2 accumulates in the blood when a person holds his or her breath. CO2 combines with water, forming carbonic acid. Carbonic acid dissociates into bicarbonate and H+, which acts to lower the pH. The drop in pH is detected by sensors that stimulate the brain, causing the person to [BREATHE]. (47) |
|
|
|
1. In the fall of 1991, sticking out of the melting snow on the crest of a high pass near the mountainous border between Italy and Austria, two Austrian hikers found a corpse. Right away it was clear the body was very old, frozen in an icy trench where he has sought shelter long ago and only now released as the ice melted. In the years since this startling find, scientists have learned a great deal about the dead man, whom they named Otzi. They know his age, his health, the shoes and clothing he wore, what he ate, and that he died from an arrow that ripped through his back. Its tip is still embedded in the back of his left shoulder. From the distribution of chemicals in his teeth and bones, we know he lived his life within 60 kilometers of where he died. |
How long ago did this Iceman die? Scientists answered this key question by measuring the degree of decay of the short-lived carbon isotope 14C in Otzi’s body. This procedure is discussed earlier in this chapter (see figure 2.8). The graph to the right displays the radioactive decay curve of the carbon isotope carbon-14 (14C); it takes 5,730 years for half of the 14C present in a sample to decay to nitrogen-14 (14N). When Otzi’s carbon isotopes were analyzed, researchers determined that the ratio of 14C to 12C (a ratio is the size of one variable relative to another), also written as the fraction 14C/12C, in Otzi’s body is 0.435 of the fraction found in tissues of a person who has recently died. (48) |
|
|
|
1. Applying Concepts. A. Variable. In the graph, what is the dependent variable? (48)
|
1. Applying Concepts. A. Variable. In the graph, what is the dependent variable? (48)
|
|
|
|
1. Applying Concepts. B. Proportion. What proportion (a proportion is the size of a variable relative to the whole) of the 14C present in Otzi’s body when he died is still there today? (48)
|
1. Applying Concepts. B. Proportion. What proportion (a proportion is the size of a variable relative to the whole) of the 14C present in Otzi’s body when he died is still there today? (48)
|
|
|
|
1. Interpreting Data. Plot this proportion on the 14C radioactive decay curve above. How many half-lives does this point represent? (48)
|
1. Interpreting Data. Plot this proportion on the 14C radioactive decay curve above. How many half-lives does this point represent? (48)
|
|
|
|
1. Making Inferences. If Otzi were indeed a recent corpse, made to look old by the harsh weather conditions found on the high mountain pass, what would you expect the ratio of 14C to 12C to be, relative to that in your own body? (48)
|
1. Making Inferences. If Otzi were indeed a recent corpse, made to look old by the harsh weather conditions found on the high mountain pass, what would you expect the ratio of 14C to 12C to be, relative to that in your own body? (48)
|
|
|
|
1. Drawing Conclusions. How old are the remains of the Iceman Otzi? (48)
|
1. Drawing Conclusions. How old are the remains of the Iceman Otzi? (48)
|
|
|
|
1. Further Analysis. A. The radioactive iodine isotope 13I decays at a half-life of eight days. Plotted on the graph above, would its radioactive decay curve be above or below that of 14C? (48)
|
1. Further Analysis. A. The radioactive iodine isotope 13I decays at a half-life of eight days. Plotted on the graph above, would its radioactive decay curve be above or below that of 14C? (48)
|
|
|
|
1. Further Analysis B. Scientists often employ the radioactive decay of Isotope potassium-40 (40K) into argon-40 (40Ar) to date old material. 40K has a half-life of 1.3 billion years. Would it be a better isotope than 14C for dating Otzi? (48)
|
1. Further Analysis B. Scientists often employ the radioactive decay of Isotope potassium-40 (40K) into argon-40 (40Ar) to date old material. 40K has a half-life of 1.3 billion years. Would it be a better isotope than 14C for dating Otzi? (48)
|
|
|
|
2.1.1A An ____ is the smallest particle that retains the chemical properties of its substance. (49) |
2.1.1A An [ATOM] is the smallest particle that retains the chemical properties of its substance. (49) |
|
|
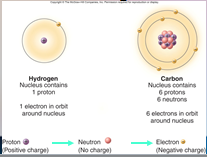
Atoms, like the carbon atom shown here from figure 2.2, contain a core nucleus of _______ and ________; _________ spin around the nucleus. (49) |
![Atoms, like the carbon atom shown here from figure 2.2, contain a core nucleus of [PROTONS] and [NEUTRONS]; [ELECTRONS] spin around the nucleus. (49)](https://images.cram.com/images/upload-flashcards/54/48/05/6544805_m.png)
Atoms, like the carbon atom shown here from figure 2.2, contain a core nucleus of [PROTONS] and [NEUTRONS]; [ELECTRONS] spin around the nucleus. (49) |
|
|
|
The number of _________ equals the number of protons in a typical atom. (49)
|
The number of [ELECTRONS] equals the number of protons in a typical atom. (49) |
|
|
|
2.1.1B The number of protons in an atom is called its atomic number. (49) |
2.1.1B The number of protons in an atom is called its [ATOMIC NUMBER]. (49) |
|
|
|
The mass that is contributed by the protons and neutrons is called the atom’s ____ ______. (49)
|
The mass that is contributed by the protons and neutrons is called the atom’s [MASS NUMBER]. (49)
|
|
|
|
All atoms that have the same ______ ______ are said to be the same element. (49)
|
All atoms that have the same [ATOMIC NUMBER]atomic number are said to be the same element. (49) |
|
|
|
2.1.2A _______ are positively charged particles, and ________ carry no charge. (49) |
2.1.2A [PROTONS] are positively charged particles, and [NEUTRONS] carry no charge. (49) |
|
|
|
a |
a |
|
|
|
Electrons are __________ charged particles that orbit around the nucleus at different ______ levels. (49) |
Electrons are [NEGATIVELY] charged particles that orbit around the nucleus at different [ENERGY] levels. (49) |
|
|
|
_________ determine the chemical behavior of an atom because they are the subatomic particles that interact with other atoms. (49)
|
[ELECTRONS] determine the chemical behavior of an atom because they are the subatomic particles that interact with other atoms. (49)
|
|
|
|
2.1.2B It takes energy to hold electrons in their orbits; this energy of position is called _________ energy. (49) |
It takes energy to hold electrons in their orbits; this energy of position is called [POTENTIAL] energy. (49) |
|
|
|
The amount of potential energy of an electron is based on its ________ from the nucleus. (49)
|
The amount of potential energy of an electron is based on its [DISTANCE] from the nucleus. (49)
|
|
|
|
2.1.2C Most electron shells hold up to _____ electrons, and atoms will undergo ________ reactions in order to fill the outermost electron shell. (49) |
Most electron shells hold up to [EIGHT] electrons, and atoms will undergo [CHEMICAL] reactions in order to fill the outermost electron shell. (49) |
|
|
|
2.2.1 Ions are atoms that have either gained one or more electrons (________ ions called anions) or lost one or more electrons (________ ions called cations). (49) |
Ions are atoms that have either gained one or more electrons ([NEGATIVE] ions called anions) or lost one or more electrons ([POSITIVE] ions called cations). (49) |
|
|
|
2.2.2 Isotopes are atoms that have the same number of _______ but differing numbers of ________. (49) |
Isotopes are atoms that have the same number of [PROTONS] but differing numbers of [NEUTRONS]. (49) |
|
|
|
Isotopes tend to be unstable and break up into other elements through a process called ___________ _____. (49)
|
Isotopes tend to be unstable and break up into other elements through a process called [RADIOACTIVE DECAY]. (49)
|
|
|
|
2.2.3 Some ________ can be used in medicine and dating fossils. (49) |
Some [ISOTOPES] can be used in medicine and dating fossils. (49) |
|
|
|
2.3.1A Molecules are _____ linked together by chemical bonds. There are three main types of chemical bonds. (49) |
Molecules are [ATOMS] linked together by chemical bonds. There are three main types of chemical bonds. (49) |
|
|
|
2.3.1B _____ _____ form when ions of opposite charge are attracted to each other. Table salt is formed by _____ _____ between positive sodium ions and negative chloride ions. (49) |
[IONIC BONDS] form when ions of opposite charge are attracted to each other. Table salt is formed by [IONIC BONDS] between positive sodium ions and negative chloride ions. (49) |
|
|
|
2.3.2 Covalent bonds form when ___ atoms share electrons, attempting to fill empty electron orbitals (integrated are, page 41). Covalent bonds are ________ when more electrons are shared. (49) |
Covalent bonds form when [TWO] atoms share electrons, attempting to fill empty electron orbitals (integrated are, page 41). Covalent bonds are [STRONGER] when more electrons are shared. (49)
|
|
|
|
2.3.3 The _____ in a polar molecule are held together by covalent bonds in which the shared electrons are ________ distributed around their nuclei, giving the molecule a slightly positive end and a slightly negative end. (49) |
The [ATOMS] in a polar molecule are held together by covalent bonds in which the shared electrons are [UNEVENLY] distributed around their nuclei, giving the molecule a slightly positive end and a slightly negative end. (49) |
|
|
|
________ _____ form when the positive end of one polar molecule is attracted to the negative end of another. (49)
|
[HYDROGEN BONDS] form when the positive end of one polar molecule is attracted to the negative end of another. (49)
|
|
|
|
2.3.4 Weak ________ ___________ called van der Waals forces can hold _____ together ___________ when they come close to each other. (49) |
Weak [CHEMICAL ATTRACTIONS] called van der Waals forces can hold [ATOMS] together [TEMPORARILY] when they come close to each other. (49) |
|
|
|
2.4.1A Water molecules are polar molecules that form ________ _____ with each other and with other polar molecules. Many of the physical properties of water are attributed to ________ _______. (49) |
Water molecules are polar molecules that form [HYDROGEN BONDS] with each other and with other polar molecules. Many of the physical properties of water are attributed to [HYDROGEN BONDING]. (49) |
|
|
|
2.4.1B Because water molecules are attracted to each other by ________ _______, a significant amount of heat energy is needed to pull the _________ apart. For this reason, water heats up ______ and holds it temperature ______. (49) |
Because water molecules are attracted to each other by [HYDROGEN BONDING], a significant amount of heat energy is needed to pull the [MOLECULES] apart. For this reason, water heats up [SLOWLY] and holds it temperature [LONGER]. (49) |
|
|
|
2.4.1C The ________ _____ that hold water molecules together become more ______ at lower temperatures, and as a result, they lock water molecules into place in solid crystal structures called ice, such as that shown here from figure 2.9. (49) |
The [HYDROGEN BONDS] that hold water molecules together become more [STABLE] at lower temperatures, and as a result, they lock water molecules into place in solid crystal structures called ice, such as that shown here from figure 2.9. (49) |
|
|
|
2.4.1D In order for water to vaporize into a gas, a significant input of ____ ______ is needed to break the hydrogen bonds. This high heat of vaporization is a property of water used by our bodies in ________________. (49) |
In order for water to vaporize into a gas, a significant input of [HEAT ENERGY] is needed to break the hydrogen bonds. This high heat of vaporization is a property of water used by our bodies in [THERMOREGULATION]. (49) |
|
|
|
2.4.1E Because water molecules are polar molecules, they will form ________ _____ with other polar molecules. If the other polar molecules are water molecules, the process is called ________. If the other polar molecules are some other substance, the process is called ________. (49) |
Because water molecules are polar molecules, they will form [HYDROGEN BONDS] with other polar molecules. If the other polar molecules are water molecules, the process is called [COHESION]. If the other polar molecules are some other substance, the process is called [ADHESION]. (49) |
|
|
|
2.4.1F When _____ molecules form hydrogen bonds with other polar molecules, they will tend to surround other polar molecules, forming a barrier around them called a _________ _____. (49) |
When [WATER] molecules form hydrogen bonds with other polar molecules, they will tend to surround other polar molecules, forming a barrier around them called a [HYDRATION SHELL]. (49) |
|
|
|
Polar molecules are said to be ___________ and are _____-_______. (49)
|
Polar molecules are said to be [HYDROPHILIC] and are [WATER-SOLUBLE]. (49)
|
|
|
|
________ molecules do not form hydrogen bonds and will _______ together when placed in water. They are said to be hydrophobic and are _____-_________. (49)
|
[NONPOLAR] molecules do not form hydrogen bonds and will [CLUSTER] together when placed in water. They are said to be hydrophobic and are [WATER-INSOLUBLE]. (49)
|
|
|
|
2.5.1 Water molecules __________, forming negatively charged hydroxide ions (OH-) and positively charged hydrogen ions (H+). This property of water is significant because the _____________ of hydrogen ions in a solution determines its pH. (49) |
Water molecules [DISSOCIATE], forming negatively charged hydroxide ions (OH-) and positively charged hydrogen ions (H+). This property of water is significant because the [CONCENTRATION] of hydrogen ions in a solution determines its pH. (49) |
|
|
|
2.5.2 A solution with a higher hydrogen ion concentration is an ____ with specific chemical properties, and a solution with a lower hydrogen ion concentration is a ____ with different chemical properties. (49) |
A solution with a higher hydrogen ion concentration is an [ACID] with specific chemical properties, and a solution with a lower hydrogen ion concentration is a [BASE] with different chemical properties. (49) |
|
|
|
2.5.3A Substances called _______ control changes in pH by taking up or releasing H+ into the solution and control pH within a range called the _________ _____. (49) |
Substances called [BUFFERS] control changes in pH by taking up or releasing H+ into the solution and control pH within a range called the [BUFFERING RANGE]. (49) |
|
|
|
2.5.3B A buffer that helps ________ blood pH in humans is an acid-base pair consisting of carbonic acid and bicarbonate. This buffering process involves a series of two reversible reactions, one that generates hydrogen ions, ________ pH, and the reverse reaction that takes up hydrogen ions from solution, __________ pH. (49) |
A buffer that helps [REGULATE] blood pH in humans is an acid-base pair consisting of carbonic acid and bicarbonate. This buffering process involves a series of two reversible reactions, one that generates hydrogen ions, [REDUCING] pH, and the reverse reaction that takes up hydrogen ions from solution, [INCREASING] pH. (49) |
|
|
|
2.1.1A The smallest particle into which a substance can be divided and still retain all of its chemical properties is: (50)
a. Matter. |
2.1.1A The smallest particle into which a substance can be divided and still retain all of its chemical properties is: (50)
a. An atom. |
|
|
|
2.1.1B The property that distinguishes an atom of one element (carbon, for example) from an atom of another element (oxygen, for example) is: (50)
a. The number of electrons. |
2.1.1B The property that distinguishes an atom of one element (carbon, for example) from an atom of another element (oxygen, for example) is: (50)
a. The number of protons. |
|
|
|
2.1.2A Which has more potential energy, an electron closer to an atom’s nucleus or an electron that is farther from the nucleus? (50) |
Which has more potential energy, an electron closer to an atom’s nucleus or an electron that is farther from the nucleus? (50) An electron farther from the atom’s nucleus has more potential energy. |
|
|
|
2.1.2B The difference between an electron shell and an electron orbital is that: (50)
a. An orbital has more mass than an electron shell. |
2.1.2B The difference between an electron shell and an electron orbital is that: (50) |
|
|
|
2.2.1A An atom that has gained or lost one or more electrons is: (50) |
2.2.1A An atom that has gained or lost one or more electrons is: (50) |
|
|
|
2.2.1B An atom with a net positive charge must have more: (50) |
2.2.1B An atom with a net positive charge must have more: (50) |
|
|
|
2.2.2A The isotopes carbon-12 and carbon-14 differ in: (50) |
2.2.2A The isotopes carbon-12 and carbon-14 differ in: (50) |
|
|
|
2.2.2B An ion differs from an isotope in that an ion: (50) |
2.2.2B An ion differs from an isotope in that an ion: (50) |
|
|
|
2.2.3A Imagine you were to find a fossilized animal bone containing one-eight the amount of carbon-14 present in earth’s atmosphere today. How long ago did the animal die? (50) |
Imagine you were to find a fossilized animal bone containing one-eight the amount of carbon-14 present in earth’s atmosphere today. How long ago did the animal die? (50) |
|
|
|
2.2.3B Explain why it is difficult to use carbon-14 dating on 100-million-year-old dinosaur fossils. (50) |
Explain why it is difficult to use carbon-14 dating on 100-million-year-old dinosaur fossils. (50) |
|
|
|
2.3.1A Atoms are held together by a force called a bond. The three principal types of chemical bonds are: (50) |
2.3.1A Atoms are held together by a force called a bond. The three principal types of chemical bonds are: (50) |
|
|
|
2.3.1B Ionic bonds: (50) |
2.3.1B Ionic bonds: (50) |
|
|
|
2.3.2A Carbon has four electrons in its outer electron shell, and thus: (50) |
2.3.2A Carbon has four electrons in its outer electron shell, and thus: (50) |
|
|
|
2.3.2B Hydrogen atoms (one valence electron) form a diatomic gas, H2, Oxygen atoms (two valence electrons) form a diatomic gas, O2. Nitrogen atoms (three valence electrons) form a diatomic gas, N2. Carbon atoms (four valence electrons) do NOT form a diatomic gas, C2. Why not? (50) |
Hydrogen atoms (one valence electron) form a diatomic gas, H2, Oxygen atoms (two valence electrons) form a diatomic gas, O2. Nitrogen atoms (three valence electrons) form a diatomic gas, N2. Carbon atoms (four valence electrons) do NOT form a diatomic gas, C2. Why not? (50) |
|
|
|
2.3.2C Explain why it is possible for a bacterium to break the triple covalent bond of N2 gas, whereas you and other animals cannot. (50) |
Explain why it is possible for a bacterium to break the triple covalent bond of N2 gas, whereas you and other animals cannot. (50) |
|
|
|
2.3.3A The partial separation of charge in the water molecule: (50) |
2.3.3A The partial separation of charge in the water molecule: (50) |
|
|
|
2.3.3B Hydrogen bonds: (50) |
2.3.3B Hydrogen bonds: (50) |
|
|
|
2.3.4 Van der Waals attractions: (50) |
2.3.4 Van der Waals attractions: (50) |
|
|
|
2.4.1A Water has some very unusual properties. These properties occur because of the: (50) |
2.4.1A Water has some very unusual properties. These properties occur because of the: (50) |
|
|
|
2.4.1B Which of the following properties of water is explained by the need for significant heat energy to break many hydrogen bonds? (50) |
2.4.1B Which of the following properties of water is explained by the need for significant heat energy to break many hydrogen bonds? (50) |
|
|
|
2.4.1C The attraction of water molecules to other water molecules is called: (50) |
2.4.1C The attraction of water molecules to other water molecules is called: (50) |
|
|
|
2.4.1D If you add ice cubes to a glass half-full of water, what happens to the level of water in the glass? Does it rise or fall? Explain why. (50) |
If you add ice cubes to a glass half-full of water, what happens to the level of water in the glass? Does it rise or fall? Explain why. (50) |
|
|
|
2.5.1 Water sometimes ionizes, a single molecule breaking apart into a hydrogen ion and a hydroxide ion. Other materials may dissociate in water, resulting in either (1) an increase of hydrogen ions or (2) a decrease of hydrogen ions in the solution. We call the results: (50) |
2.5.1 Water sometimes ionizes, a single molecule breaking apart into a hydrogen ion and a hydroxide ion. Other materials may dissociate in water, resulting in either (1) an increase of hydrogen ions or (2) a decrease of hydrogen ions in the solution. We call the results: (50) |
|
|
|
2.5.2 A solution with a high concentration of hydrogen ions: (50) |
2.5.2 A solution with a high concentration of hydrogen ions: (50) |
|
|
|
2.5.3 Which of the following is not true about buffers? (50) |
2.5.3 Which of the following is not true about buffers? (50) |
|
|
|
Formed by living organisms and consist of a carbon-based core with special groups attached. A molecule of the kind normally found in living systems; are usually composed of carbon atoms in rings or long chains, to which are attached other atoms of such elements as hydrogen, oxygen, and nitrogen. |
1. Organic Molecules (52) |
|
|
|
An extremely large molecule. Refers specifically to carbohydrates, lipids, proteins, and nucleic acids. |
1. Macromolecules (52) |
|
|
|
Simple molecules that can join together to form polymers. |
1. Monomers (52) |
|
|
|
A large molecule formed of long chains of similar molecules called monomers.
|
1. Polymer (52) |
|
|
|
A chemical reaction that builds up molecules by losing water molecules. It is a type of condensation reaction in which monomers join together into polymers while losing water molecules. This process is carried out by losing (-OH) from one of the monomers and (H) from another monomer. The two unstable monomers join together, and the (-OH) and (H) combine forming water (H2O).
|
1. Dehydration Synthesis (53) |
|
|
|
A protein capable of speeding up specific chemical reactions by lowering the energy required to activate or start the reaction but that remains unaltered in the process.
|
1. Enzymes (53)
|
|
|
|
The chemical breakdown of a compound due to reaction with water.
|
1. Hydrolysis (53)
|
|
|
|
A long chain of amino acids linked end to end by peptide bonds. Because the 20 amino acids that occur in proteins have side groups with very different chemical properties, the function and shape of a protein are critically affected by its particular sequence of amino acids.
|
1. Proteins (54) |
|
|
|
The subunit structure from which proteins are produced, consisting of a central carbon atom with a carboxyl group (COOH), an amino group (-NH2), a hydrogen, and a side group (R group); only the side group differs from one amino acid to another.
|
1. Amino Acids (54)
|
|
|
|
A covalent bond linking two amino acids. Formed when the positive (amino, or NH2) group at one end and a negative (carboxyl, or COOH) group at the other end undergo a chemical reaction and lose a molecule of water.
|
1. Peptide Bond (54)
|
|
|
|
A general term for a long chain of amino acids linked end to end by peptide bonds. A protein is a long, complex ___________.
|
1. Polypeptides (54) |
|
|
|
The characteristic sequence of amino acids forming a protein or polypeptide chain, considered as the most basic element of its structure.
|
1. Primary Structure (56)
|
|
|
|
The local three-dimensional structure of sheets, helices, or other forms adopted by a polynucleotide or polypeptide chain, due to electrostatic attraction between neighboring residues.
|
1. Secondary Structure (56)
|
|
|
|
The overall three-dimensional structure resulting from folding and covalent cross-linking of a protein or polynucleotide molecule.
|
1. Tertiary Structure (56) |
|
|
|
The structure that is formed by the joining together of two or more proteins or nucleic acids. The functions of the proteins and nucleic acids are only expressed correctly when they are joined together.
|
1. Quaternary Structure (56)
|
|
|
|
To destroy the characteristic properties of (a protein or other biological macromolecule) by heat, acidity, or other effects that disrupt its molecular conformation.
|
1. Denatured (57)
|
|
|
|
The enzyme-mediated process in which the subunits of polymers are positioned so that their bonds undergo chemical reactions.
|
1. Catalysis (57) |
|
|
|
Molecular __________ are ________ that assist the non-covalent folding or unfolding and the assembly or disassembly of other macromolecular structures.
|
1. Chaperone Proteins (58)
|
|
|
|
A small proteinaceous infectious disease-causing agent that is believed to be the smallest infectious particle. A _____ is neither bacterial nor fungal nor viral and contains no genetic material. ______ have been held responsible for a number of degenerative brain diseases, including mad cow disease, Creutzfeldt-Jakob disease, fatal familial insomnia, kuru, and an unusual form of hereditary dementia known as Gertsmann-Straeussler-Scheinker disease.
|
1. Prions (58) |
|
|
|
Also known as prion diseases, are a group of progressive conditions (encephalopathies) that affect the brain and nervous system of many animals, including humans. These diseases include scrapie in sheep, “mad cow” disease in cattle, and kuru and Creutzfeldt-Jakob disease in humans.
|
1. Transmissible Spongiform Encephalopathies (TSEs) (59) |
|
|
|
A degenerative neurologic disease of cattle, thought to be caused by infection-causing agents called prions, in which brain tissues deteriorate and take on a spongy appearance, resulting in abnormal behaviors and loss of muscle control. A variant form of Creutzfeldt-Jakob disease is transmitted to humans through the eating of infected cattle tissue. Also called bovine spongiform encephalopathy.
|
1. Mad Cow Disease (59)
|
|
|
|
A nucleotide polymer. A long chain of nucleotides. Chief types are deoxyribonucleic acid (DNA), which is double-stranded, and ribonucleic acid (RNA), which is typically single-stranded.
|
1. Nucleic Acids (60)
|
|
|
|
A single unit of a nucleic acid, composed of a phosphate, a five-carbon sugar (either ribose or deoxyribose), and a purine or a pyrimidine.
|
1. Nucleotides (60) |
|
|
|
A sequence of nucleotides, as in DNA or RNA, bound into a chain. ______________. A linear polymer containing an indefinite, usually large, number of nucleotides linked from one ribose or deoxyribose to another by phosphoric residues.
|
1. Polynucleotide Chains (60)
|
|
|
|
The basic storage vehicle or central plan of heredity information. It is stored as a sequence of nucleotides in a linear nucleotide polymer. Two of the polymers wind around each other like the outside and inside rails of a circular staircase.
|
1. Deoxyribonucleic Acid (DNA) (60) |
|
|
|
A class of nucleic acids characterized by the presence of the sugar ribose and the pyrimidine uracil; includes mRNA, tRNA, rRNA, and siRNA.
|
1. Ribonucleic Acid (RNA) (60) |
|
|
|
A pair of parallel helices intertwined about a common axis, especially that in the structure of the DNA molecule.
|
1. Double Helix (61)
|
|
|
|
An organic compound consisting of a chain or ring of carbon atoms to which hydrogen and oxygen atoms are attached in a ratio of approximately 1:2:1. A compound of carbon, hydrogen, and oxygen having the generalized formula (CH2O)n, where n is the number of carbon atoms.
|
1. Carbohydrates (63)
|
|
|
|
A simple sugar. |
1. Monosaccharides (63) |
|
|
|
A sugar formed by linking two monosaccharide molecules together. Sucrose (table sugar) is a disaccharide formed by linking a molecule of glucose to a molecule of fructose.
|
1. Disaccharide (63)
|
|
|
|
A sugar polymer. A carbohydrate composed of many monosaccharide sugar subunits linked together in a long chain.
|
1. Polysaccharides (63)
|
|
|
|
The glucose polysaccharide that plants use to store energy. An odorless tasteless white substance occurring widely in plant tissue and obtained chiefly from cereals and potatoes. It is a polysaccharide that functions as a carbohydrate store and is an important constituent of the human diet.
|
1. Starch (63)
|
|
|
|
In animals, energy is stored in this, an insoluble macromolecule formed of long glucose polysaccharides that are highly branched. A substance deposited in bodily tissues as a store of carbohydrates. It is a polysaccharide that forms glucose on hydrolysis.
|
1. Glycogen (63)
|
|
|
|
Animals also use glucose chains as building materials, linking the subunits together in different orientations not recognized by most enzymes. A fibrous substance consisting of polysaccharides and forming the major constituent in the exoskeleton of arthropods and the cell walls of fungi.
|
1. Chitin (63)
|
|
|
|
Plants also use glucose chains as building materials, linking the subunits together in different orientations not recognized by most enzymes. An insoluble substance that is the main constituent of plant cell walls and of vegetable fibers such as cotton. It is a polysaccharide consisting of chains of glucose monomers.
|
1. Cellulose (63)
|
|
|
|
A loosely defined group of molecules that are insoluble in water but soluble in oil. Oils such as olive, corn, and coconut are lipids, as well as waxes, such as beeswax and earwax.
|
1. Lipids (65)
|
|
|
|
A long chain of carbon and hydrogen atoms (called a hydrocarbon) ending in a carboxyl (-COOH) group. A carboxylic acid consisting of a hydrocarbon chain and a terminal carboxyl group, especially any of those occurring as esters in fats and oils.
|
1. Fatty Acid (65) |
|
|
|
An ester formed from glycerol and three fatty acid groups. _____________ are the main constituents of natural fats and oils, and high concentrations in the blood indicate an elevated risk of stroke.
|
1. Triglyceride (65)
|
|
|
|
Containing the greatest possible number of hydrogen atoms, and so having no carbon–carbon double or triple bonds.
|
1. Saturated (65)
|
|
|
|
Fats composed of fatty acids that have double bonds between one or more pairs of carbon atoms contain fewer than the maximum number of hydrogen atoms. Having carbon–carbon double or triple bonds and therefore not containing the greatest possible number of hydrogen atoms for the number of carbons. Denoting fats containing a high proportion of fatty acid molecules with at least one double bond, considered to be healthier in the diet than saturated fats.
|
1. Unsaturated (65) |
|
|
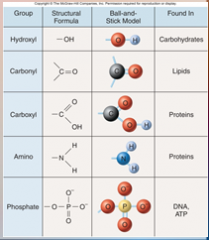
Macromolecules: Organic Molecules – formed by ______ organisms and consist of a carbon-based core with ‘_______ groups’ attached. |
![Macromolecules: Organic Molecules – formed by [LIVING] organisms and consist of a carbon-based core with ‘[SPECIAL GROUPS]’ attached.](https://images.cram.com/images/upload-flashcards/54/59/81/6545981_m.png)
Macromolecules: Organic Molecules – formed by [LIVING] organisms and consist of a carbon-based core with ‘[SPECIAL GROUPS]’ attached. |
|
|
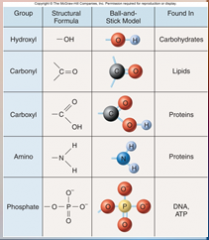
Macromolecules: Functional Groups – ‘special groups’ with special chemical properties that tend to act as _____ during chemical reactions. |
![Macromolecules: Functional Groups – ‘special groups’ with special chemical properties that tend to act as [UNITS] during chemical reactions.](https://images.cram.com/images/upload-flashcards/54/59/96/6545996_m.png)
Macromolecules: Functional Groups – ‘special groups’ with special chemical properties that tend to act as [UNITS] during chemical reactions. |
|
|

Macromolecules: Macromolecules – very large macromolecules that make up a lot of the body (________, nucleic acids, _____________, lipids). |
![Macromolecules: Macromolecules – very large macromolecules that make up a lot of the body ([PROTEINS], nucleic acids, [CARBOHYDRATES], lipids).](https://images.cram.com/images/upload-flashcards/54/60/08/6546008_m.png)
Macromolecules: Macromolecules – very large macromolecules that make up a lot of the body ([PROTEINS], nucleic acids, [CARBOHYDRATES], lipids). |
|
|

Polymers are made of Monomers: Monomer – _______ _____ attached like a train. |
![Polymers are made of Monomers: Monomer – [SMALLER PARTS] attached like a train.](https://images.cram.com/images/upload-flashcards/54/60/17/6546017_m.png)
Polymers are made of Monomers: Monomer – [SMALLER PARTS] attached like a train. |
|
|
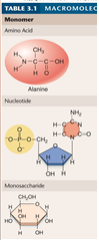
Polymers are made of Monomers: Polymer – ________ build of monomers. |
![Polymers are made of Monomers: Polymer – [MOLECULE] build of monomers.](https://images.cram.com/images/upload-flashcards/54/60/47/6546047_m.png)
Polymers are made of Monomers: Polymer – [MOLECULE] build of monomers. |
|
|
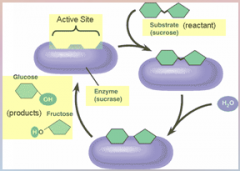
Polymers are made of Monomers: Bonds can be formed or broken using special proteins (_______) to facilitate the ___________ of molecules. |
![Polymers are made of Monomers: Bonds can be formed or broken using special proteins ([ENZYMES]) to facilitate the [POSITIONING] of molecules.](https://images.cram.com/images/upload-flashcards/54/60/56/6546056_m.png)
Polymers are made of Monomers: Bonds can be formed or broken using special proteins ([ENZYMES]) to facilitate the [POSITIONING] of molecules. |
|
|
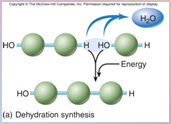
Polymers are made of Monomers: Dehydration Synthesis – ________ ____ formed by the removal of OH and H groups (molecule of water). |
![Polymers are made of Monomers: Dehydration Synthesis – [COVALENT BOND] formed by the removal of OH and H groups (molecule of water).](https://images.cram.com/images/upload-flashcards/54/60/68/6546068_m.png)
Polymers are made of Monomers: Dehydration Synthesis – [COVALENT BOND] formed by the removal of OH and H groups (molecule of water). |
|
|
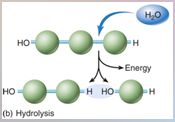
__________ – breaking of a polymer by adding H to one subunit and OH to another thus breaking the covalent bond. |
![[HYDROLYSIS] – breaking of a polymer by adding H to one subunit and OH to another thus breaking the covalent bond.](https://images.cram.com/images/upload-flashcards/54/60/77/6546077_m.png)
[HYDROLYSIS] – breaking of a polymer by adding H to one subunit and OH to another thus breaking the covalent bond. |
|
|
Proteins: Proteins – complex biological macromolecules (________) with a big role in the body. |
![Proteins: Proteins – complex biological macromolecules ([POLYMERS]) with a big role in the body.](https://images.cram.com/images/upload-flashcards/54/60/86/6546086_m.png)
Proteins: Proteins – complex biological macromolecules ([POLYMERS]) with a big role in the body. |
|
|
Proteins: Enzymes – _____ the energy required to initiation a particular ________ reaction. |
![Proteins: Enzymes – [LOWER] the energy required to initiation a particular [CHEMICAL] reaction.](https://images.cram.com/images/upload-flashcards/54/60/92/6546092_m.png)
Proteins: Enzymes – [LOWER] the energy required to initiation a particular [CHEMICAL] reaction. |
|
|
Proteins: Structural Roles – Collagen makes up _________, _____ and _______ made of collagen. |
![Proteins: Structural Roles – Collagen makes up [CARTILAGE], [BONES] and [TENDONS] made of collagen.](https://images.cram.com/images/upload-flashcards/54/60/98/6546098_m.png)
Proteins: Structural Roles – Collagen makes up [CARTILAGE], [BONES] and [TENDONS] made of collagen. |
|
|

Proteins: Structural Roles – _______ forms hair, horns or rhinoceros and feathers. |
![Proteins: Structural Roles – [KERATIN] forms hair, horns or rhinoceros and feathers.](https://images.cram.com/images/upload-flashcards/54/61/04/6546104_m.png)
Proteins: Structural Roles – [KERATIN] forms hair, horns or rhinoceros and feathers. |
|
|
Proteins: Structural Roles – Chemical messengers. (?) |
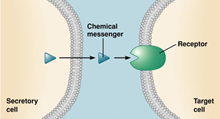
Proteins: Structural Roles – Chemical messengers. (?) |
|
|
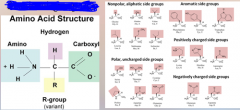
Proteins: Amino Acids are monomers with a ______ base structure (amino and carboxyl groups) and a __________ group (R). |
![Proteins: Amino Acids are monomers with a [SIMPLE] base structure (amino and carboxyl groups) and a [FUNCTIONAL] group (R).](https://images.cram.com/images/upload-flashcards/54/61/19/6546119_m.png)
Proteins: Amino Acids are monomers with a [SIMPLE] base structure (amino and carboxyl groups) and a [FUNCTIONAL] group (R). |
|
|
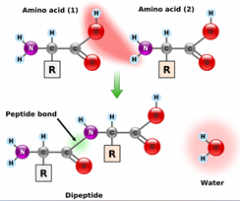
Proteins: Peptide Bond – covalent bond that links two _____ _____ via dehydration synthesis. |
![Proteins: Peptide Bond – covalent bond that links two [AMINO ACIDS] via dehydration synthesis.](https://images.cram.com/images/upload-flashcards/54/61/25/6546125_m.png)
Proteins: Peptide Bond – covalent bond that links two [AMINO ACIDS] via dehydration synthesis. |
|
|
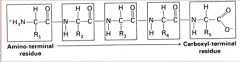
Proteins: Polypeptide – long chains of amino acids linked by _______ _____. |
![Proteins: Polypeptide – long chains of amino acids linked by [PEPTIDE BONDS].](https://images.cram.com/images/upload-flashcards/54/61/34/6546134_m.png)
Proteins: Polypeptide – long chains of amino acids linked by [PEPTIDE BONDS]. |
|
|
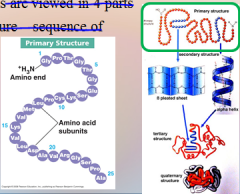
Proteins: Protein structures are viewed in 4 parts – Primary Structure – sequence of _____ _____. |
![Proteins: Protein structures are viewed in 4 parts – Primary Structure – sequence of [AMINO ACIDS].](https://images.cram.com/images/upload-flashcards/54/61/49/6546149_m.png)
Proteins: Protein structures are viewed in 4 parts – Primary Structure – sequence of [AMINO ACIDS]. |
|
|
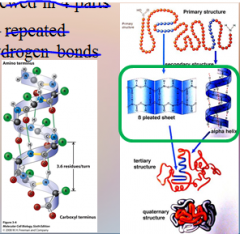
Proteins: Protein structures are viewed in 4 parts – Secondary Structure – repeated ________ formed by hydrogen bonds between R groups (α-_____). |
![Proteins: Protein structures are viewed in 4 parts – Secondary Structure – repeated [PATTERNS] formed by hydrogen bonds between R groups (α-[HELIX]).](https://images.cram.com/images/upload-flashcards/54/61/55/6546155_m.png)
Proteins: Protein structures are viewed in 4 parts – Secondary Structure – repeated [PATTERNS] formed by hydrogen bonds between R groups (α-[HELIX]). |
|
|

Proteins: Protein structures are viewed in 4 parts – Secondary Structure – repeated patterns formed by ________ _____ between R groups (β-_______ _____). |
![Proteins: Protein structures are viewed in 4 parts – Secondary Structure – repeated patterns formed by [HYDROGEN BONDS] between R groups (β-[PLEATED SHEET]).](https://images.cram.com/images/upload-flashcards/54/62/63/6546263_m.png)
Proteins: Protein structures are viewed in 4 parts – Secondary Structure – repeated patterns formed by [HYDROGEN BONDS] between R groups (β-[PLEATED SHEET]). |
|
|
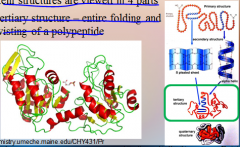
Proteins: Protein structures are viewed in 4 parts – Tertiary Structure – entire _______ and ________ of a polypeptide. |
![Proteins: Protein structures are viewed in 4 parts – Tertiary Structure – entire [FOLDING] and [TWISTING] of a polypeptide.](https://images.cram.com/images/upload-flashcards/54/62/87/6546287_m.png)
Proteins: Protein structures are viewed in 4 parts – Tertiary Structure – entire [FOLDING] and [TWISTING] of a polypeptide. |
|
|
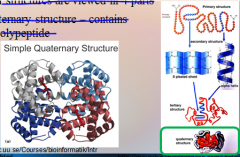
Proteins: Protein structures are viewed in 4 parts – Quaternary structure – contains >1 ___________. |
![Proteins: Protein structures are viewed in 4 parts – Quaternary structure – contains >1 [POLYPEPTIDE].](https://images.cram.com/images/upload-flashcards/54/63/05/6546305_m.png)
Proteins: Protein structures are viewed in 4 parts – Quaternary structure – contains >1 [POLYPEPTIDE]. |
|
|
Proteins: Protein _______ is a result of the polar, watery, environment of the cell and the _____ formed between R groups. |
![Proteins: Protein [FOLDING] is a result of the polar, watery, environment of the cell and the [BONDS] formed between R groups.](https://images.cram.com/images/upload-flashcards/54/63/20/6546320_m.png)
Proteins: Protein [FOLDING] is a result of the polar, watery, environment of the cell and the [BONDS] formed between R groups. |
|
|
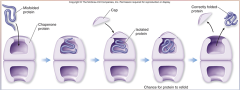
Proteins: Some proteins do not fold properly and need the aid of ________ ________ to help it fold correctly. |
![Proteins: Some proteins do not fold properly and need the aid of [CHAPERONE PROTEINS] to help it fold correctly.](https://images.cram.com/images/upload-flashcards/54/63/32/6546332_m.png)
Proteins: Some proteins do not fold properly and need the aid of [CHAPERONE PROTEINS] to help it fold correctly. |
|
|
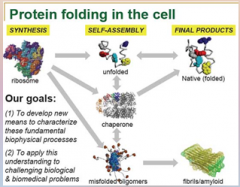
Proteins: Proteins that do not fold properly can lead to _______. |
![Proteins: Proteins that do not fold properly can lead to [DISEASE].](https://images.cram.com/images/upload-flashcards/54/63/41/6546341_m.png)
Proteins: Proteins that do not fold properly can lead to [DISEASE]. |
|
|
|
Proteins: Proteins that do not fold properly can lead to disease – __________ _______ – could be in part due to chaperone protein ____________ leading to amyloid protein clumping in brain cells. |
Proteins: Proteins that do not fold properly can lead to disease – [ALZHEIMER’S DISEASE] – could be in part due to chaperone protein [DEFICIENCIES] leading to amyloid protein clumping in brain cells. |
|
|
|
Proteins: Proteins that do not fold properly can lead to disease – ___ ___ _______ and ___________-_____ _______ – caused by misfolded brain proteins (prions) that induce other brain prion proteins to misfold, thus forming a _____ ________ of misfolding killing more brain cells.
|
Proteins: Proteins that do not fold properly can lead to disease – [MAD COW DISEASE] and [CREUTZFELDT-JACOB DISEASE] – caused by misfolded brain proteins (prions) that induce other brain prion proteins to misfold, thus forming a [CHAIN REACTION] of misfolding killing more brain cells. |
|
|
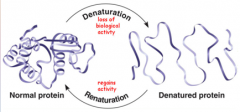
Proteins: Denaturing (_________ of a protein) occurs when the cellular environment _______ altering hydrogen bonds. |
![Proteins: Denaturing ([UNFOLDING] of a protein) occurs when the cellular environment [CHANGES] altering hydrogen bonds.](https://images.cram.com/images/upload-flashcards/54/63/77/6546377_m.png)
Proteins: Denaturing ([UNFOLDING] of a protein) occurs when the cellular environment [CHANGES] altering hydrogen bonds. |
|
|

Proteins: 1OF 5 BIOLOGICAL THEMES – Protein _________ determines ________. Enzymes fold into globular structures to make an active site where a ________ takes place acting as a catalyst. |
![Proteins: 1OF 5 BIOLOGICAL THEMES – Protein [STRUCTURE] determines [FUNCTION]. Enzymes fold into globular structures to make an active site where a [REACTION] takes place acting as a catalyst.](https://images.cram.com/images/upload-flashcards/54/63/86/6546386_m.png)
Proteins: 1OF 5 BIOLOGICAL THEMES – Protein [STRUCTURE] determines [FUNCTION]. Enzymes fold into globular structures to make an active site where a [REACTION] takes place acting as a catalyst. |
|
|
Proteins: 1OF 5 BIOLOGICAL THEMES – Protein structure determines function. Many structural proteins form long ______ like the contractile proteins of muscle cells. |
![Proteins: 1OF 5 BIOLOGICAL THEMES – Protein structure determines function. Many structural proteins form long [CABLES] like the contractile proteins of muscle cells.](https://images.cram.com/images/upload-flashcards/54/73/76/6547376_m.png)
Proteins: 1OF 5 BIOLOGICAL THEMES – Protein structure determines function. Many structural proteins form long [CABLES] like the contractile proteins of muscle cells. |
|
|
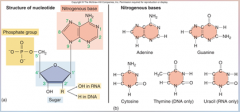
Nucleic Acids: _______ _____ are polymers that act as the information storage device of cells. This polymer is made of monomers called ___________ that are organized into 5 parts. |
![Nucleic Acids: [NUCLEIC ACIDS] are polymers that act as the information storage device of cells. This polymer is made of monomers called [NUCLEOTIDES] that are organized into 5 parts.](https://images.cram.com/images/upload-flashcards/54/73/85/6547385_m.png)
Nucleic Acids: [NUCLEIC ACIDS] are polymers that act as the information storage device of cells. This polymer is made of monomers called [NUCLEOTIDES] that are organized into 5 parts. |
|
|
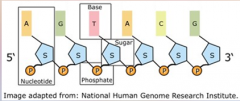
Nucleic Acids: A ______________ chain is formed when the 5-carbon sugars are linked with a _________ group. |
![Nucleic Acids: A [POLYNUCLEOTIDE] chain is formed when the 5-carbon sugars are linked with a [PHOSPHATE] group.](https://images.cram.com/images/upload-flashcards/54/74/12/6547412_m.png)
Nucleic Acids: A [POLYNUCLEOTIDE] chain is formed when the 5-carbon sugars are linked with a [PHOSPHATE] group. |
|
|
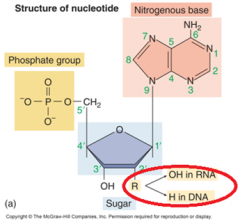
Nucleic Acids: ________________ ____ (DNA) vs. ___________ ____ (RNA) |
![Nucleic Acids: [DEOXYRIBONUCLEIC ACID] (DNA) vs. [RIBONUCLEIC ACID] (RNA)](https://images.cram.com/images/upload-flashcards/54/74/06/6547406_m.jpg)
Nucleic Acids: [DEOXYRIBONUCLEIC ACID] (DNA) vs. [RIBONUCLEIC ACID] (RNA) |
|
|
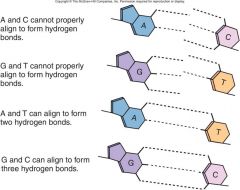
Nucleic Acids: DNA is a ______ _____. |
![Nucleic Acids: DNA is a [DOUBLE HELIX].](https://images.cram.com/images/upload-flashcards/54/74/18/6547418_m.jpg)
Nucleic Acids: DNA is a [DOUBLE HELIX]. |
|
|
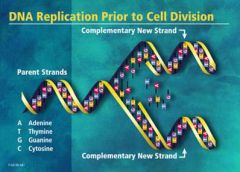
Nucleic Acids: 1 OF 5 CHARACTERISTICS FOR BEING ALIVE – DNA is the __________ information that is passed on to offspring. |
![Nucleic Acids: 1 OF 5 CHARACTERISTICS FOR BEING ALIVE – DNA is the [HEREDITARY] information that is passed on to offspring.](https://images.cram.com/images/upload-flashcards/54/74/42/6547442_m.jpg)
Nucleic Acids: 1 OF 5 CHARACTERISTICS FOR BEING ALIVE – DNA is the [HEREDITARY] information that is passed on to offspring. |
|
|
|
Carbohydrates: Carbohydrates are ________ that make up the structural framework of cells and play a critical role in storing ______.
|
Carbohydrates: Carbohydrates are [POLYMERS] that make up the structural framework of cells and play a critical role in storing [ENERGY]. |
|
|
|
Carbohydrates: Simple Carbohydrate – small ________ or dimmers.
|
Carbohydrates: Simple Carbohydrate – small [MONOMERS] or dimmers.
|
|
|
|
Carbohydrates: Complex Carbohydrate – long ________.
|
Carbohydrates: Complex Carbohydrate – long [POLYMERS].
|
|
|
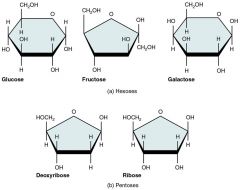
Carbohydrates: Simple Carbohydrate – ______________ or “simple sugar.” |
![Carbohydrates: Simple Carbohydrate – [MONOSACCHARIDE] or “simple sugar.”](https://images.cram.com/images/upload-flashcards/54/74/54/6547454_m.jpg)
Carbohydrates: Simple Carbohydrate – [MONOSACCHARIDE] or “simple sugar.” |
|
|
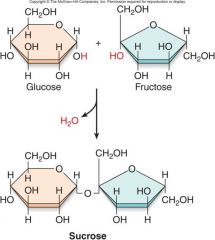
Carbohydrates: Simple Carbohydrate – ____________, dimer. |
![Carbohydrates: Simple Carbohydrate – [DISACCHARIDE], dimer.](https://images.cram.com/images/upload-flashcards/54/74/81/6547481_m.png)
Carbohydrates: Simple Carbohydrate – [DISACCHARIDE], dimer. |
|
|
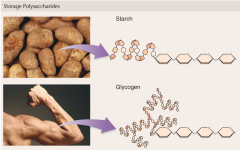
Carbohydrates: Complex Carbohydrates – polysaccharides (______ and ________). |
![Carbohydrates: Complex Carbohydrates – polysaccharides ([STARCH] and [GLYCOGEN]).](https://images.cram.com/images/upload-flashcards/54/74/90/6547490_m.png)
Carbohydrates: Complex Carbohydrates – polysaccharides ([STARCH] and [GLYCOGEN]). |
|
|
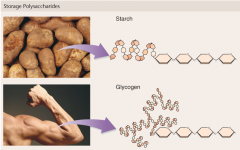
Carbohydrates: Complex Carbohydrates – polysaccharides – Starch – glucose polysaccharide that ______ use to store energy. |
![Carbohydrates: Complex Carbohydrates – polysaccharides – Starch – glucose polysaccharide that [PLANTS] use to store energy.](https://images.cram.com/images/upload-flashcards/54/74/96/6547496_m.png)
Carbohydrates: Complex Carbohydrates – polysaccharides – Starch – glucose polysaccharide that [PLANTS] use to store energy. |
|
|
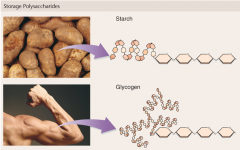
Carbohydrates: Complex Carbohydrates – polysaccharides – Glycogen – highly branched soluble glucose polysaccharide that _______ use to store energy. |
![Carbohydrates: Complex Carbohydrates – polysaccharides – Glycogen – highly branched soluble glucose polysaccharide that [ANIMALS] use to store energy.](https://images.cram.com/images/upload-flashcards/54/75/08/6547508_m.png)
Carbohydrates: Complex Carbohydrates – polysaccharides – Glycogen – highly branched soluble glucose polysaccharide that [ANIMALS] use to store energy. |
|
|
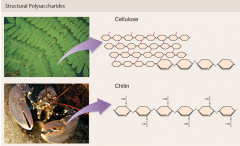
Carbohydrates: Complex Carbohydrates – polysaccharides – Chitin (______) and Cellulose (_______) – glucose chains used as building materials, linking the subunits together in different ____________ not recognized by most enzymes. |
![Carbohydrates: Complex Carbohydrates – polysaccharides – Chitin ([PLANTS]) and Cellulose ([ANIMALS]) – glucose chains used as building materials, linking the subunits together in different [ORIENTATIONS] not recognized by most enzymes.](https://images.cram.com/images/upload-flashcards/54/75/29/6547529_m.png)
Carbohydrates: Complex Carbohydrates – polysaccharides – Chitin ([PLANTS]) and Cellulose ([ANIMALS]) – glucose chains used as building materials, linking the subunits together in different [ORIENTATIONS] not recognized by most enzymes. |
|
|
|
Lipids: Lipids are nonpolar polymers, thus _________ in water, used for ____-term energy storage and other functions within the cell.
|
Lipids: Lipids are nonpolar polymers, thus [INSOLUBLE] in water, used for [LONG]-term energy storage and other functions within the cell. |
|
|
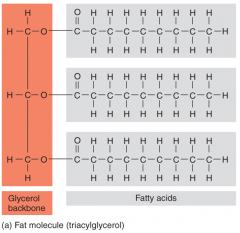
Lipids: Fats (_____________) are lipids made of fatty acids (long chain of carbon and hydrogen) and a ________ backbone. |
![Lipids: Fats ([TRIGLYCERIDES]) are lipids made of fatty acids (long chain of carbon and hydrogen) and a [GLYCEROL] backbone.](https://images.cram.com/images/upload-flashcards/54/75/32/6547532_m.png)
Lipids: Fats ([TRIGLYCERIDES]) are lipids made of fatty acids (long chain of carbon and hydrogen) and a [GLYCEROL] backbone. |
|
|
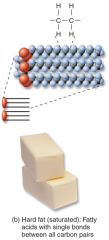
Lipids: Saturated fats are fatty acids with all ________ carbon atoms forming ________ bonds with 2 hydrogen atoms contain the maximum number of hydrogen atoms. |
![Lipids: Saturated fats are fatty acids with all [INTERNAL] carbon atoms forming [COVALENT] bonds with 2 hydrogen atoms contain the maximum number of hydrogen atoms.](https://images.cram.com/images/upload-flashcards/54/75/38/6547538_m.png)
Lipids: Saturated fats are fatty acids with all [INTERNAL] carbon atoms forming [COVALENT] bonds with 2 hydrogen atoms contain the maximum number of hydrogen atoms. |
|
|
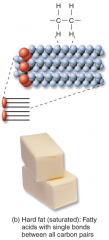
Lipids: Saturated fats are _____ at room temperature. |
![Lipids: Saturated fats are [SOLID] at room temperature.](https://images.cram.com/images/upload-flashcards/54/75/47/6547547_m.png)
Lipids: Saturated fats are [SOLID] at room temperature. |
|
|
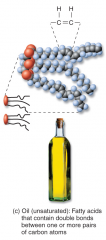
Lipids: Unsaturated fats are fatty acids with some ________ carbons having ______ bonds, thus contain fewer than the maximum number of hydrogen atoms. |
![Lipids: Unsaturated fats are fatty acids with some [INTERNAL] carbons having [DOUBLE] bonds, thus contain fewer than the maximum number of hydrogen atoms.](https://images.cram.com/images/upload-flashcards/54/75/56/6547556_m.png)
Lipids: Unsaturated fats are fatty acids with some [INTERNAL] carbons having [DOUBLE] bonds, thus contain fewer than the maximum number of hydrogen atoms. |
|
|
Lipids: Most unsaturated fats are ______ at room temperature due to the _____ formed by the double bonds. |
![Lipids: Most unsaturated fats are [LIQUID] at room temperature due to the [KINKS] formed by the double bonds.](https://images.cram.com/images/upload-flashcards/54/75/62/6547562_m.png)
Lipids: Most unsaturated fats are [LIQUID] at room temperature due to the [KINKS] formed by the double bonds. |
|
|

Lipids: Some ___________ fatty acids have artificially hydrogenated (addition of more hydrogen), making them more _________, to extend the shelf-life of a product. |
![Lipids: Some [UNSATURATED] fatty acids have artificially hydrogenated (addition of more hydrogen), making them more [SATURATED], to extend the shelf-life of a product.](https://images.cram.com/images/upload-flashcards/54/75/71/6547571_m.jpg)
Lipids: Some [UNSATURATED] fatty acids have artificially hydrogenated (addition of more hydrogen), making them more [SATURATED], to extend the shelf-life of a product. |
|
|

Lipids: Trans fats can sometimes be created by ____________ in which some of the double bonds are less kinked than naturally occurring unsaturated fats. |
![Lipids: Trans fats can sometimes be created by [HYDROGENATED] in which some of the ______ bonds are less kinked than naturally occurring unsaturated fats.](https://images.cram.com/images/upload-flashcards/54/75/80/6547580_m.jpg)
Lipids: Trans fats can sometimes be created by [HYDROGENATED] in which some of the ______ bonds are less kinked than naturally occurring unsaturated fats. |
|
|
Lipids: Eating trans fats and saturated fats can increase the risk of _____ _______. |
![Lipids: Eating trans fats and saturated fats can increase the risk of [HEART DISEASE].](https://images.cram.com/images/upload-flashcards/54/75/86/6547586_m.jpg)
Lipids: Eating trans fats and saturated fats can increase the risk of [HEART DISEASE]. |
|
|
Lipids: ________ are lipids that consist of ________ ring structures – Testosterone (male sex hormone). |
![Lipids: [STEROIDS] are lipids that consist of [MULTIPLE] ring structures – Testosterone (male sex hormone).](https://images.cram.com/images/upload-flashcards/54/75/95/6547595_m.png)
Lipids: [STEROIDS] are lipids that consist of [MULTIPLE] ring structures – Testosterone (male sex hormone). |
|
|
Lipids: Steroids are ______ that consist of multiple ring structures – _________ (female sex hormone). |
![Lipids: Steroids are [LIPIDS] that consist of multiple ring structures – [ESTRADIOL] (female sex hormone).](https://images.cram.com/images/upload-flashcards/54/76/07/6547607_m.png)
Lipids: Steroids are [LIPIDS] that consist of multiple ring structures – [ESTRADIOL] (female sex hormone). |
|
|
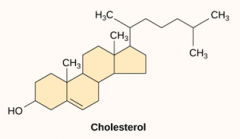
Lipids: Steroids are lipids that consist of multiple ____ __________ – ___________. |
![Lipids: Steroids are lipids that consist of multiple [RING STRUCTURES] – [CHOLESTEROL].](https://images.cram.com/images/upload-flashcards/54/76/25/6547625_m.png)
Lipids: Steroids are lipids that consist of multiple [RING STRUCTURES] – [CHOLESTEROL]. |
|
|

Lipids: Phospholipids are modified _______________ molecules where one of the carbons in the glycerol backbone bonds to a phosphate group instead of a third _____ ____. |
![Lipids: Phospholipids are modified [TRIACYLGLYCEROL] molecules where one of the carbons in the glycerol backbone bonds to a phosphate group instead of a third [Fatty Acid].](https://images.cram.com/images/upload-flashcards/54/77/90/6547790_m.png)
Lipids: Phospholipids are modified [TRIACYLGLYCEROL] molecules where one of the carbons in the glycerol backbone bonds to a phosphate group instead of a third [Fatty Acid]. |
|
|
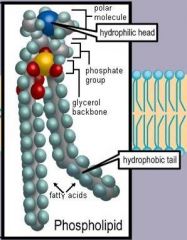
Lipids: The phosphate group gives the phospholipid molecule an overall shape of a _____ head with 2 ________ tails. Group together to form a lipid bilayer. |
![Lipids: The phosphate group gives the phospholipid molecule an overall shape of a [POLAR] head with 2 [NONPOLAR] tails. Group together to form a lipid bilayer.](https://images.cram.com/images/upload-flashcards/54/77/06/6547706_m.jpg)
Lipids: The phosphate group gives the phospholipid molecule an overall shape of a [POLAR] head with 2 [NONPOLAR] tails. Group together to form a lipid bilayer. |
|
|
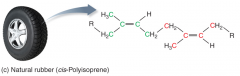
Lipids: Other important biological lipids are ______, _____ and ________ (such as chlorophyll that makes plants green and the retinal that your eyes use to detect light). |
![Lipids: Other important biological lipids are [RUBBER], [WAXES] and [PIGMENTS] (such as chlorophyll that makes plants green and the retinal that your eyes use to detect light).](https://images.cram.com/images/upload-flashcards/54/77/36/6547736_m.png)
Lipids: Other important biological lipids are [RUBBER], [WAXES] and [PIGMENTS] (such as chlorophyll that makes plants green and the retinal that your eyes use to detect light). |
|
|
|
The bodies of organisms contain thousands of different kinds of _________ and _____. Organisms obtain many of these molecules from their ____________ and from what they consume. (52) |
The bodies of organisms contain thousands of different kinds of [MOLECULES] and [ATOMS]. Organisms obtain many of these molecules from their [SURROUNDINGS] and from what they consume. (52) |
|
|
|
You might be familiar with some of the substances listed on nutritional labels, such as the one shown in figure 3.1. But what do the words on these labels mean? Some of them are names of ________ (atoms, see chapter 2), such as calcium and iron, and others are ________. (52) |
You might be familiar with some of the substances listed on nutritional labels, such as the one shown in figure 3.1. But what do the words on these labels mean? Some of them are names of [MINERALS] (atoms, see chapter 2), such as calcium and iron, and others are [VITAMINS]. (52) |
|
|
|
Still others (what’s on nutrition labels) are the subject of this chapter: large molecules that make up the bodies of organisms and are found in our food, such as proteins, carbohydrates (including sugars), and lipids (including, fats, trans fats, saturated fats, and cholesterol). These molecules, called _______ molecules, are formed by living organisms and consist of a carbon-based core with special groups attached. These groups of atoms have special chemical properties and are referred to as __________ groups. (52) |
Still others (what’s on nutrition labels) are the subject of this chapter: large molecules that make up the bodies of organisms and are found in our food, such as proteins, carbohydrates (including sugars), and lipids (including, fats, trans fats, saturated fats, and cholesterol). These molecules, called [ORGANIC] molecules, are formed by living organisms and consist of a carbon-based core with special groups attached. These groups of atoms have special chemical properties and are referred to as [FUNCTIONAL] groups. (52) |
|
|
|
Functional groups tend to act as _____ during chemical reactions and to confer specific chemical properties on the _________ that possess them. Five principal functional groups are listed in figure 3.2; the last column indicates the types of organic molecules that contain these functional groups. (52) |
Functional groups tend to act as [UNITS] during chemical reactions and to confer specific chemical properties on the [MOLECULES] that possess them. [FIVE] principal functional groups are listed in figure 3.2; the last column indicates the types of organic molecules that contain these functional groups. (52) |
|
|
|
The bodies of organisms contain thousands of different kinds of organic molecules, but much of the body is made of just four kinds: ________, _______ _____, _____________, and ______. Called ______________ because they can be very large, these four are the building materials of cells, the “bricks and mortar” that make up the bodies of cells and the machinery that runs within them. (52) |
The bodies of organisms contain thousands of different kinds of organic molecules, but much of the body is made of just four kinds: [PROTEINS], [NUCLEIC ACIDS], [CARBOHYDRATES], and [LIPIDS]. Called [MACROMOLECULES] because they can be very large, these four are the building materials of cells, the “bricks and mortar” that make up the bodies of cells and the machinery that runs within them. (52) |
|
|
|
The body’s macromolecules are assembled by sticking smaller bits, called ________, together, much as a train is built by linking railcars together. A molecule built up of long chains of similar subunits is called a _______. Table 3.1 lists the monomers in the first column that make up the polymers that are the basis for many ________ structures. (52) |
The body’s macromolecules are assembled by sticking smaller bits, called [MONOMERS], together, much as a train is built by linking railcars together. A molecule built up of long chains of similar subunits is called a [POLYMER]. Table 3.1 lists the monomers in the first column that make up the polymers that are the basis for many [CELLULAR] structures. (52) |
|
|
|
Figure 3.2 – Five principal functional groups. These functional groups can be ___________ from one molecule to another and are common in organic molecules. (52) |
Figure 3.2 – Five principal functional groups. These functional groups can be [TRANSFERRED] from one molecule to another and are common in organic molecules. (52) |
|
|
|
Figure 3.1 – What’s in a nutritional label? ____, ___________, _____________, ______, and ________ are just some of the molecules found in popcorn and discussed in this chapter. (52) |
Figure 3.1 – What’s in a nutritional label? [FATS], [CHOLESTEROL], [CARBOHYDRATES], [SUGARS], and [PROTEINS] are just some of the molecules found in popcorn and discussed in this chapter. (52) |
|
|
|
The four different kinds of macromolecules (________, _______ _____, _____________, and ______) are all put together in the same way: a ________ ____ is formed between two subunits in which a hydroxyl group (OH) is removed from one subunit and a hydrogen (H) is removed from the other. (53) |
The four different kinds of macromolecules ([PROTEINS], [NUCLEIC ACIDS], [CARBOHYDRATES], and [LIPIDS]) are all put together in the same way: a [COVALENT BOND] is formed between two subunits in which a hydroxyl group (OH) is removed from one subunit and a hydrogen (H) is removed from the other. (53) |
|
|
|
This process (illustrated in figure 3.3a) is called dehydration synthesis because, in effect, the removal of the OH and H groups (highlighted by the blue oval) constitutes removal of a ________ of water – the word dehydration means “taking away water.” (53) |
This process (illustrated in figure 3.3a) is called dehydration synthesis because, in effect, the removal of the OH and H groups (highlighted by the blue oval) constitutes removal of a [MOLECULE] of water – the word dehydration means “taking away water.” (53) |
|
|
|
Dehydration synthesis requires the help of a special class of proteins called _______ to facilitate the positioning of the molecules so that the correct chemical _____ are stressed and broken. (53) |
Dehydration synthesis requires the help of a special class of proteins called [ENZYMES] to facilitate the positioning of the molecules so that the correct chemical [BONDS] are stressed and broken. (53) |
|
|
|
The process of tearing down a molecule, such as the protein or fat contained in food that is consumed, is essentially the _______ of dehydration synthesis: Instead of removing a water molecule, one is added. When a water molecule comes in, as shown in figure 3.3b, a hydrogen becomes attached to one subunit and a hydroxyl to another, and the covalent bond is broken. The breaking up of a polymer in this way is called __________. (53) |
The process of tearing down a molecule, such as the protein or fat contained in food that is consumed, is essentially the [REVERSE] of dehydration synthesis: Instead of removing a water molecule, one is added. When a water molecule comes in, as shown in figure 3.3b, a hydrogen becomes attached to one subunit and a hydroxyl to another, and the covalent bond is broken. The breaking up of a polymer in this way is called [HYDROLYSIS]. (53) |
|
|
|
Key Learning Outcome 3.1 – Macromolecules are formed by linking subunits together into long chains, ________ a water molecule as each link is formed. In contrast, macromolecules are broken down into their subunits by hydrolysis reactions, the ________ of water molecules. (53) |
Key Learning Outcome 3.1 – Macromolecules are formed by linking subunits together into long chains, [REMOVING] a water molecule as each link is formed. In contrast, macromolecules are broken down into their subunits by hydrolysis reactions, the [ADDITION] of water molecules. (53) |
|
|
|
Figure 3.3 – Dehydration and hydrolysis. (a) Biological molecules are formed by linking subunits. The ________ bond between subunits is formed in dehydration synthesis, a process during which a water molecule is __________. (b) Breaking such a bond requires the addition of a water molecule, a reaction called __________. (53) |
Figure 3.3 – Dehydration and hydrolysis. (a) Biological molecules are formed by linking subunits. The [COVALENT] bond between subunits is formed in dehydration synthesis, a process during which a water molecule is [ELIMINATED]. (b) Breaking such a bond requires the addition of a water molecule, a reaction called [HYDROLYSIS]. (53) |
|
|
|
Complex macromolecules called proteins are a major group of biological macromolecules within the bodies of _________. Figure 3.4 presents an overview of the wide-ranging functions of proteins. |
Complex macromolecules called proteins are a major group of biological macromolecules within the bodies of [ORGANISMS]. Figure 3.4 presents an overview of the wide-ranging functions of proteins. |
|
|
|
Despite their diverse functions, all ________ have the same basic structure: a long polymer chain made of subunits called _____ _____. (54) |
Despite their diverse functions, all [PROTEINS] have the same basic structure: a long polymer chain made of subunits called [AMINO ACIDS]. (54) |
|
|
|
Amino acids are _____ molecules with a simple basic structure: a central carbon atom to which an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (H), and a functional group, designated “R,” are ______. (54) |
Amino acids are [SMALL] molecules with a simple basic structure: a central carbon atom to which an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (H), and a functional group, designated “R,” are [BONDED]. (54) |
|
|
|
There are 20 common kinds of amino acids that differ from one another by the ________ of their functional R group. (54) |
There are 20 common kinds of amino acids that differ from one another by the [IDENTITY] of their functional R group. (54) |
|
|
|
The 20 amino acids are classified into ____ general groups, with representative amino acids shown in figure 3.5 (their R groups are highlighted in white). (54) |
The 20 amino acids are classified into [FOUR] general groups, with representative amino acids shown in figure 3.5 (their R groups are highlighted in white). (54) |
|
|
|
Six of the amino acids are ________, differing chiefly in size – the most bulky contain ring structures (like asparagine in the upper right), and these differ from one another in the strength of their ________. (54) |
Six of the amino acids are [NONPOLAR], differing chiefly in size – the most bulky contain ring structures (like asparagine in the upper right), and these differ from one another in the strength of their [POLARITY]. (54) |
|
|
|
Five more (amino acids) are polar and are capable of ________ to a charged form (like aspartic acid in the lower left). (54) |
Five more (amino acids) are polar and are capable of [IONIZING] to a charged form (like aspartic acid in the lower left). (54) |
|
|
|
The remaining three possess special ________ groups (like the white highlighted area of proline in the lower right) that are important in forming links between chains or in forming kinks in their ______. (54) |
The remaining three possess special [CHEMICAL] groups (like the white highlighted area of proline in the lower right) that are important in forming links between chains or in forming kinks in their [SHAPES]. (54) |
|
|
|
The ________ of the R groups is important to the proper folding of the protein into its functional shape. (54) |
The [POLARITY] of the R groups is important to the proper folding of the protein into its functional shape. (54) |
|
|
|
An individual protein is made by linking specific _____ _____ together in a particular order, just as a word is made by linking specific letters of the alphabet together in a particular order. (54) |
An individual protein is made by linking specific [AMINO ACIDS] together in a particular order, just as a word is made by linking specific letters of the alphabet together in a particular order. (54) |
|
|
|
The covalent bond linking two amino acids together is called a _______ bond and forms by ___________ _________. (54) |
The covalent bond linking two amino acids together is called a [PEPTIDE] bond and forms by [DEHYDRATION SYNTHESIS]. (54) |
|
|
|
Recall from section 3.1 that in dehydration synthesis, water is formed as a by-product of the ________. You can see in figure 3.6 that a water molecule is released as the peptide bond forms. (54) |
Recall from section 3.1 that in dehydration synthesis, water is formed as a by-product of the [REACTION]. You can see in figure 3.6 that a water molecule is released as the peptide bond forms. (54) |
|
|
|
Long chains of amino acids linked by peptide bonds are called ____________. (54) |
Long chains of amino acids linked by peptide bonds are called [POLYPEPTIDES]. (54) |
|
|
|
Functional polypeptides are more commonly called ________. (54) |
Functional polypeptides are more commonly called [PROTEINS]. (54) |
|
|
|
Figure 3.5 – Examples of amino acids. There are four general groups of amino acids that differ in their __________ ______ (highlighted in white). (54) |
Figure 3.5 – Examples of amino acids. There are four general groups of amino acids that differ in their [FUNCTIONAL GROUPS] (highlighted in white). (54) |
|
|
|
Figure 3.6 – The formation of a peptide bond. Every amino acid has the same basic _________, with an amino group (-NH2) at one end and a carboxyl group (-COOH) at the other. The only variable is the functional, or “_,” _____. (54) |
Figure 3.6 – The formation of a peptide bond. Every amino acid has the same basic [STRUCTURE], with an amino group (-NH2) at one end and a carboxyl group (-COOH) at the other. The only variable is the functional, or “[R,” GROUP]. (54) |
|
|
|
Amino acids are linked by ___________ _________ to form peptide bonds. Chains of amino acids linked in this way are called polypeptides and are the basic structural components of ________. (54) |
Amino acids are linked by [DEHYDRATION SYNTHESIS] to form peptide bonds. Chains of amino acids linked in this way are called polypeptides and are the basic structural components of [PROTEINS]. (54) |
|
|
|
Figure 3.4 – Some of the different types of proteins. (a) _______: Globular proteins called _______ play a key role in many chemical reactions. (55) |
Some of the different types of proteins. (a) [ENZYMES]: Globular proteins called [ENZYMES] play a key role in many chemical reactions. (55) |
|
|
|
Figure 3.4 – Some of the different types of proteins. (b) _________ proteins: Red blood cells contain the protein hemoglobin, which transports oxygen and carbon dioxide in the body. (55) |
Figure 3.4 – Some of the different types of proteins. (b) [TRANSPORT proteins: Red blood cells contain the protein hemoglobin, which transports oxygen and carbon dioxide in the body. (55) |
|
|
|
Figure 3.4 – Some of the different types of proteins. (c) __________ proteins (collagen): Collagen is present in bones, tendons, and cartilage. (55) |
Figure 3.4 – Some of the different types of proteins. (c) [STRUCTURAL] proteins (collagen): Collagen is present in bones, tendons, and cartilage. (55) |
|
|
|
Figure 3.4 – Some of the different types of proteins. (d) __________ proteins (keratin): Keratin forms hair, nails, feathers, and components of horns. (55) |
Figure 3.4 – Some of the different types of proteins. (d) [STRUCTURAL] proteins (keratin): Keratin forms hair, nails, feathers, and components of horns. (55) |
|
|
|
Figure 3.4 – Some of the different types of proteins. (e) _________ proteins: White blood cells destroy cells without the proper identity proteins and make antibody proteins that attack invaders. (55) |
Figure 3.4 – Some of the different types of proteins. (e) [DEFENSIVE] proteins: White blood cells destroy cells without the proper identity proteins and make antibody proteins that attack invaders. (55) |
|
|
|
Figure 3.4 – Some of the different types of proteins. (f) ___________ proteins: Proteins called actin and myosin are present in muscles. (55) |
Figure 3.4 – Some of the different types of proteins. (f) [CONTRACTILE] proteins: Proteins called actin and myosin are present in muscles. (55) |
|
|
|
Some proteins form ____, ____ fibers, whereas others are globular, their strands ______ __ and folded back on themselves. (56) |
Some proteins form [LONG, THIN] fibers, whereas others are globular, their strands [COILED UP] and folded back on themselves. (56) |
|
|
|
The _____ of a protein is very important because it determines the protein’s function. There are four general levels of protein structure: primary, secondary, tertiary, and quaternary; all are ultimately determined by the ________ of amino acids. (56) |
The [SHAPE] of a protein is very important because it determines the protein’s function. There are four general levels of protein structure: primary, secondary, tertiary, and quaternary; all are ultimately determined by the [SEQUENCE] of amino acids. (56) |
|
|
|
Primary Structure – The sequence of amino acids of a ___________ chain is termed the ___________’_ primary structure. (56) |
Primary Structure – The sequence of amino acids of a [POLYPEPTIDE] chain is termed the [POLYPEPTIDE’S] primary structure. (56) |
|
|
|
The amino acids are linked together by _______ bonds, forming long chains like a “beaded strand.” (56) |
The amino acids are linked together by [PEPTIDE] bonds, forming long chains like a “beaded strand.” (56) |
|
|
|
The primary structure of a protein, the ________ of its amino acids, determines all other levels of protein structure. (56) |
The primary structure of a protein, the [SEQUENCE] of its amino acids, determines all other levels of protein structure. (56) |
|
|
|
Because amino acids can be assembled in any ________, a great diversity of proteins is possible. (56) |
Because amino acids can be assembled in any [SEQUENCE], a great diversity of proteins is possible. (56) |
|
|
|
Secondary Structure – Hydrogen bonds forming between different parts of the polypeptide chain _________ the folding of the polypeptide. As you can see, these ___________ hydrogen bonds, indicated by red dotted lines, do not involve the R groups themselves but rather the polypeptide backbone. This initial folding is called the secondary structure of a protein. (56) |
Secondary Structure – Hydrogen bonds forming between different parts of the polypeptide chain [STABILIZE] the folding of the polypeptide. As you can see, these [STABILIZING] hydrogen bonds, indicated by red dotted lines, do not involve the R groups themselves but rather the polypeptide backbone. This initial folding is called the secondary structure of a protein. (56) |
|
|
|
Hydrogen bonding within this secondary structure can fold the polypeptide into _____, called α-helices, and ______, called β-pleated sheets. (56) |
Hydrogen bonding within this secondary structure can fold the polypeptide into [COILS], called α-helices, and [SHEETS], called β-pleated sheets. (56) |
|
|
|
________ Structure – Because some of the amino acids are nonpolar, a polypeptide chain folds up in water, which is very polar, pushing nonpolar amino acid functional groups from the watery environment. (56) |
[TERTIARY] Structure – Because some of the amino acids are nonpolar, a polypeptide chain folds up in water, which is very polar, pushing nonpolar amino acid functional groups from the watery environment. (56) |
|
|
|
The final three-dimensional shape, or tertiary structure, of the protein, folded and twisted in the case of a globular molecule, is determined by exactly _____ in a polypeptide chain the nonpolar amino acids occur. (56) |
The final three-dimensional shape, or tertiary structure, of the protein, folded and twisted in the case of a globular molecule, is determined by exactly [WHERE] in a polypeptide chain the nonpolar amino acids occur. (56) |
|
|
|
Quaternary Structure – When a protein is composed of more than ___ polypeptide chain, the spatial arrangement of the several _________ chains is called the quaternary structure of the protein. For example, four subunits make up the quaternary structure of the protein hemoglobin. (56) |
Quaternary Structure – When a protein is composed of more than [ONE] polypeptide chain, the spatial arrangement of the several [COMPONENT] chains is called the quaternary structure of the protein. For example, four subunits make up the quaternary structure of the protein hemoglobin. (56) |
|
|
|
The _____ nature of the watery environment in the cell influences how the polypeptide folds into the functional protein. A protein is folded in such a way that allows it to carry out its ________. (57) |
The [POLAR] nature of the watery environment in the cell influences how the polypeptide folds into the functional protein. A protein is folded in such a way that allows it to carry out its [FUNCTION]. (57) |
|
|
|
If the polar nature of the protein’s environment changes by either increasing ___________ or lowering __, both of which alter hydrogen bonding, the protein may unfold, as in the lower right of the figure. When this happens the protein is said to be _________. (57) |
If the polar nature of the protein’s environment changes by either increasing [TEMPERATURE] or lowering [pH], both of which alter hydrogen bonding, the protein may unfold, as in the lower right of the figure. When this happens the protein is said to be [DENATURED]. (57) |
|
|
|
When the _____ nature of the solvent is reestablished, some proteins may spontaneously refold. When proteins are denatured, they usually lose their ability to ________ properly. That is the rationale behind traditional methods of salt-curing and pickling food. (57) |
When the [POLAR] nature of the solvent is reestablished, some proteins may spontaneously refold. When proteins are denatured, they usually lose their ability to [FUNCTION] properly. That is the rationale behind traditional methods of salt-curing and pickling food. (57) |
|
|
|
Prior to the ready availability of refrigerators and freezers, the only practical way to keep microorganisms from growing in food was top keep the food in a solution containing a high concentration of salt or vinegar, which _________ proteins in the microorganisms and kept them from growing on the food. (57) |
Prior to the ready availability of refrigerators and freezers, the only practical way to keep microorganisms from growing in food was top keep the food in a solution containing a high concentration of salt or vinegar, which [DENATURED] proteins in the microorganisms and kept them from growing on the food. (57) |
|
|
|
Because the primary structure of a protein, its ________ of amino acids, determines how the protein folds into its functional shape, a change in the identity of even one _____ ____ can have profound effects on a protein’s ability to function properly. (57)
|
Because the primary structure of a protein, its [SEQUENCE] of amino acids, determines how the protein folds into its functional shape, a change in the identity of even one [AMINO ACID] can have profound effects on a protein’s ability to function properly. (57)
|
|
|
|
_______ are globular proteins that have three-dimensional shapes. (57)
|
[ENZYMES] are globular proteins that have three-dimensional shapes. (57)
|
|
|
|
For enzymes to function properly, they need to ____ correctly. (57)
|
For enzymes to function properly, they need to [FOLD] correctly. (57)
|
|
|
|
Enzymes have grooves or depressions that precisely fit a particular sugar or other chemical (like the red molecule binding to the enzyme to the right); once in the groove, the chemical is encouraged to undergo a ________ – often, one of its chemical bonds is stressed as the chemical is bent by the enzyme, like a foot in a flexing shoe. This process of enhancing chemical reactions is called _________, and proteins are the catalytic agents of cells, determining what chemical processes take place and where and when. (57)
|
Enzymes have grooves or depressions that precisely fit a particular sugar or other chemical (like the red molecule binding to the enzyme to the right); once in the groove, the chemical is encouraged to undergo a [REACTION] – often, one of its chemical bonds is stressed as the chemical is bent by the enzyme, like a foot in a flexing shoe. This process of enhancing chemical reactions is called [CATALYSIS], and proteins are the catalytic agents of cells, determining what chemical processes take place and where and when. (57)
|
|
|
|
Many structural proteins form long cables that have architectural roles in cells, providing ________ and determining _____. Cells contain a network of protein cables that maintain the shape of the cell and function in ____________ materials throughout the cell (figure 3.7). (57)
|
Many structural proteins form long cables that have architectural roles in cells, providing [STRENGTH] and determining [SHAPE]. Cells contain a network of protein cables that maintain the shape of the cell and function in [TRANSPORTING] materials throughout the cell (figure 3.7). (57)
|
|
|
|
___________ proteins function in muscle contraction, which is the shortening of a muscle. (57)
|
[CONTRACTILE] proteins function in muscle contraction, which is the shortening of a muscle. (57)
|
|
|
|
A muscle ________ when two proteins that are anchored on opposite ends of a muscle fiber slide past each other, bringing the ends of the fiber closer together. (57)
|
A muscle [SHORTENS] when two proteins that are anchored on opposite ends of a muscle fiber slide past each other, bringing the ends of the fiber closer together. (57)
|
|
|
|
Figure 3.7 – Protein structure determines ________. Fluorescently labeled structural proteins within a cell. (57)
|
Figure 3.7 – Protein structure determines [FUNCTION]. Fluorescently labeled structural proteins within a cell. (57)
|
|
|
|
How does a protein fold into a specific shape? As just discussed, ________ amino acids play a key role. Until recently, investigators thought that newly made proteins fold spontaneously as hydrophobic interactions with water shove nonpolar amino acids into the protein interior. We now know this is too simple a view. Proteins can fold into many different ways that trial and error would simply take too long. In addition, as the open chain folds its way toward its final form, ________ “sticky” interior portions are exposed during intermediate stages. If these intermediate forms are placed in a test tube in the same protein environment that occurs in a cell, they stick to other unwanted protein partners, forming a gluey mess. (58) |
How does a protein fold into a specific shape? As just discussed, [NONPOLAR] amino acids play a key role. Until recently, investigators thought that newly made proteins fold spontaneously as hydrophobic interactions with water shove nonpolar amino acids into the protein interior. We now know this is too simple a view. Proteins can fold into many different ways that trial and error would simply take too long. In addition, as the open chain folds its way toward its final form, [NONPOLAR] “sticky” interior portions are exposed during intermediate stages. If these intermediate forms are placed in a test tube in the same protein environment that occurs in a cell, they stick to other unwanted protein partners, forming a gluey mess. (58) |
|
|
|
How do cells avoid this? A vital clue came in studies of unusual _________ (changes in DNA) that prevented viruses from replicating in bacterial cells – it turned out the virus proteins could not fold properly! Further study revealed that normal cells contain special proteins called chaperone proteins that help new proteins fold correctly. When the bacterial gene encoding its chaperone protein is disabled by ________, the bacteria die, clogged with lumps of incorrectly folded proteins. Fully 30% of the bacteria’s proteins fail to fold into the right shape. (58)
|
How do cells avoid this? A vital clue came in studies of unusual [MUTATIONS] (changes in DNA) that prevented viruses from replicating in bacterial cells – it turned out the virus proteins could not fold properly! Further study revealed that normal cells contain special proteins called chaperone proteins that help new proteins fold correctly. When the bacterial gene encoding its chaperone protein is disabled by [MUTATION], the bacteria die, clogged with lumps of incorrectly folded proteins. Fully 30% of the bacteria’s proteins fail to fold into the right shape. (58)
|
|
|
|
Molecular biologists have now identified more than 17 kinds of proteins that act as molecular __________. Many are heat shock proteins, produced in greater amounts if a cell is exposed to elevated temperature; high temperatures cause proteins to unfold, and heat shock _________ proteins help the cell’s proteins refold. (58)
|
Molecular biologists have now identified more than 17 kinds of proteins that act as molecular [CHAPERONES]. Many are heat shock proteins, produced in greater amounts if a cell is exposed to elevated temperature; high temperatures cause proteins to unfold, and heat shock [CHAPERONE] proteins help the cell’s proteins refold. (58)
|
|
|
|
To understand how a _________ works, examine figure 3.8 closely. The misfolded protein enters inside the _________. There, in a way not clearly understood, the visiting protein is induced to unfold and then refold again, before it leaves. You can see in the third panel of the diagram the protein has unfolded into a long polypeptide chain. In the fourth panel, the polypeptide chain has then refolded into a different shape. The _________ protein has in this way “rescued” a protein that was caught in a wrongly folded state and given it another chance to fold correctly. To demonstrate this rescue capability, investigators “fed” a deliberately misfolded protein malate dehydrogenase to _________ proteins; the malate dehydrogenase was rescued, refolding to its active shape. (58)
|
To understand how a [CHAPERONE] works, examine figure 3.8 closely. The misfolded protein enters inside the [CHAPERONE]. There, in a way not clearly understood, the visiting protein is induced to unfold and then refold again, before it leaves. You can see in the third panel of the diagram the protein has unfolded into a long polypeptide chain. In the fourth panel, the polypeptide chain has then refolded into a different shape. The [CHAPERONE] protein has in this way “rescued” a protein that was caught in a wrongly folded state and given it another chance to fold correctly. To demonstrate this rescue capability, investigators “fed” a deliberately misfolded protein malate dehydrogenase to [CHAPERONE] proteins; the malate dehydrogenase was rescued, refolding to its active shape. (58)
|
|
|
|
There are tantalizing suggestions that chaperone protein deficiencies may play a role in _________’_ disease. By failing to facilitate the intricate folding of key proteins, the deficiency leads to the amyloid protein clumping in brain cells characteristic of the disease. ___ ___ disease and the similar human disorder called variant ___________-_____ disease are both caused by misfolded brain proteins called prions. The misfolded prions induce other brain prion proteins to misfold in turn, creating a chain reaction of misfolding that kills ever more brain cells, leading to progressive loss of brain function and eventual death. (58)
|
There are tantalizing suggestions that chaperone protein deficiencies may play a role in [ALZHEIMER’S] disease. By failing to facilitate the intricate folding of key proteins, the deficiency leads to the amyloid protein clumping in brain cells characteristic of the disease. [MAD COW] disease and the similar human disorder called variant [CREUTZFELDT-JACOB] disease are both caused by misfolded brain proteins called prions. The misfolded prions induce other brain prion proteins to misfold in turn, creating a chain reaction of misfolding that kills ever more brain cells, leading to progressive loss of brain function and eventual death. (58)
|
|
|
|
Key Learning Outcome 3.2 – Proteins are made up of chains of amino acids that ____ into complex shapes. The sequence of its amino acids determines a protein’s function. Chaperone proteins help newly produced proteins to ____ properly. (58)
|
Key Learning Outcome 3.2 – Proteins are made up of chains of amino acids that [FOLD] into complex shapes. The sequence of its amino acids determines a protein’s function. Chaperone proteins help newly produced proteins to [FOLD] properly. (58)
|
|
|
|
Figure 3.8 – How one type of chaperone protein works. This barrel-shaped chaperone protein is a ____ _____ protein, produced in elevated amounts at high temperature. An incorrectly folded protein enters one chamber of the barrel, and a cap seals the chamber and confines the protein. The isolated protein is now prevented from aggregating with other misfolded proteins, and it has a chance to refold properly. After a short time, the protein is ejected, folded or unfolded, and the cycle can repeat itself. (58)
|
Figure 3.8 – How one type of chaperone protein works. This barrel-shaped chaperone protein is a [HEAT SHOCK] protein, produced in elevated amounts at high temperature. An incorrectly folded protein enters one chamber of the barrel, and a cap seals the chamber and confines the protein. The isolated protein is now prevented from aggregating with other misfolded proteins, and it has a chance to refold properly. After a short time, the protein is ejected, folded or unfolded, and the cycle can repeat itself. (58)
|
|
|
|
Prions and Mad Cow Disease – For decades, scientists have been fascinated by a peculiar group of fatal brain diseases. These diseases have the unusual property that they are transmissible from one individual to another, but it is years and often decades before the disease is detected in infected individuals. The brains of these individuals develop numerous small cavities as neurons die, producing a marked spongy appearance, as shown here in the photo. Called _____________ __________ ________________ (TSEs), these diseases include scrapie in sheep, “mad cow” disease in cattle, and kuru and Creutzfeldt-Jakob disease in humans. (59)
|
Prions and Mad Cow Disease – For decades, scientists have been fascinated by a peculiar group of fatal brain diseases. These diseases have the unusual property that they are transmissible from one individual to another, but it is years and often decades before the disease is detected in infected individuals. The brains of these individuals develop numerous small cavities as neurons die, producing a marked spongy appearance, as shown here in the photo. Called [TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES] (TSEs), these diseases include scrapie in sheep, “mad cow” disease in cattle, and kuru and Creutzfeldt-Jakob disease in humans. (59)
|
|
|
|
Prions and Mad Cow Disease – TSEs can be transmitted between individuals of a _______ by injecting infected brain tissue into a recipient animal’s brain. TSEs can also spread via tissue transplants and, apparently, food. Kuru was common in the Fore people of Papua New Guinea when they practiced ritual cannibalism, literally eating the brains of infected individuals. Mad cow disease spread widely among the cattle herds of England in the 1990s because cows were fed bone meal prepared from sheep carcasses to increase the protein content of their diet. Like the Fore, the British cattle were literally eating the tissue of sheep that had died of scrapie. (59)
|
Prions and Mad Cow Disease – TSEs can be transmitted between individuals of a [SPECIES] by injecting infected brain tissue into a recipient animal’s brain. TSEs can also spread via tissue transplants and, apparently, food. Kuru was common in the Fore people of Papua New Guinea when they practiced ritual cannibalism, literally eating the brains of infected individuals. Mad cow disease spread widely among the cattle herds of England in the 1990s because cows were fed bone meal prepared from sheep carcasses to increase the protein content of their diet. Like the Fore, the British cattle were literally eating the tissue of sheep that had died of scrapie. (59)
|
|
|
|
Prions and Mad Cow Disease – In the 1960s, British researchers T. _____ and J. ________ noted that infectious TSE preparations remained infectious even after exposure to radiation that would destroy DNA or RNA. They suggested that the infectious agent was a protein. Perhaps, they speculated, the protein usually preferred one folding pattern but could sometimes misfold and then ________ other proteins to do the same, the misfolding spreading like a chain reaction. This heretical suggestion was not accepted by the scientific community, as it violates a key tenet of molecular biology: only DNA or RNA act as hereditary material, transmitting information from one generation to the next. (59)
|
Prions and Mad Cow Disease – In the 1960s, British researchers T. [ALPER] and J. [GRIGGITH] noted that infectious TSE preparations remained infectious even after exposure to radiation that would destroy DNA or RNA. They suggested that the infectious agent was a protein. Perhaps, they speculated, the protein usually preferred one folding pattern but could sometimes misfold and then [CATALYZE] other proteins to do the same, the misfolding spreading like a chain reaction. This heretical suggestion was not accepted by the scientific community, as it violates a key tenet of molecular biology: only DNA or RNA act as hereditary material, transmitting information from one generation to the next. (59)
|
|
|
|
Prions and Mad Cow Disease – In the early 1970s, physician Stanley ________, moved by the death of a patient from Creutzfeldt-Jakob disease, began to study TSEs. ________ became fascinated with Alper and Griffith’s hypothesis. Try as he might, ________ could find no evidence of nucleic acids, bacteria, or viruses in the infectious TSE preparation. He concluded, as Alper and Griffith had, that the infectious agent was a protein, which he named a prion, for “proteinaceous infectious particle.” (59)
|
Prions and Mad Cow Disease – In the early 1970s, physician Stanley [PRUSINER], moved by the death of a patient from Creutzfeldt-Jakob disease, began to study TSEs. [PRUSINER] became fascinated with Alper and Griffith’s hypothesis. Try as he might, [PRUSINER] could find no evidence of nucleic acids, bacteria, or viruses in the infectious TSE preparation. He concluded, as Alper and Griffith had, that the infectious agent was a protein, which he named a prion, for “proteinaceous infectious particle.” (59) |
|
|
|
Prions and Mad Cow Disease – Prusiner went on to [ISOLATE] a distinctive prion protein, and for two decades, he continued to amass evidence that prions play a key role in triggering TSEs. The scientific community resisted Prusiner’s renegade conclusions, but eventually experiments done in Prusiner’s and other laboratories began to convince many. For example, when Prusiner injected prions of different abnormal conformations into several different hosts, these hosts developed prions with the same abnormal conformations as the parent prions. In another important experiment, Charles Weissmann showed that mice genetically engineered to lack Prusiner’s prion protein are immune to TSE infection. However, if brain tissue with the prion protein is grafted into the mice, the grafted tissue – but not the rest of the brain – can then be infected with TSE. In 1997, Prusiner was awarded the Nobel Prize in Physiology or Medicine for his work on prions. (59)
|
Prions and Mad Cow Disease – Prusiner went on to [ISOLATE] a distinctive prion protein, and for two decades, he continued to amass evidence that prions play a key role in triggering TSEs. The scientific community resisted Prusiner’s renegade conclusions, but eventually experiments done in Prusiner’s and other laboratories began to convince many. For example, when Prusiner injected prions of different abnormal conformations into several different hosts, these hosts developed prions with the same abnormal conformations as the parent prions. In another important experiment, Charles Weissmann showed that mice genetically engineered to lack Prusiner’s prion protein are immune to TSE infection. However, if brain tissue with the prion protein is grafted into the mice, the grafted tissue – but not the rest of the brain – can then be infected with TSE. In 1997, Prusiner was awarded the Nobel Prize in Physiology or Medicine for his work on prions. (59)
|
|
|
|
Prions and Mad Cow Disease – Can Humans Catch Mad Cow Disease by Eating Infected Meat? – Many scientists are becoming worried that ______ may be transmitting such diseases to humans. Specifically, they worry that _____-caused bovine spongiform encephalopathy (BSE), a brain disease in cows commonly known as mad cow disease, may infect humans and produce a similar fatal disorder, variant Creutzfeldt-Jakob disease (vCJD). (59)
|
Prions and Mad Cow Disease – Can Humans Catch Mad Cow Disease by Eating Infected Meat? – Many scientists are becoming worried that [PRIONS] may be transmitting such diseases to humans. Specifically, they worry that [PRION]-caused bovine spongiform encephalopathy (BSE), a brain disease in cows commonly known as mad cow disease, may infect humans and produce a similar fatal disorder, variant Creutzfeldt-Jakob disease (vCJD). (59)
|
|
|
|
In March 1996, an outbreak of ___ ___ _______ in Britain, with over 80,000 cows infected, created widespread concern. BSE, a degeneration of the brain caused by prions, appears to have entered the British cattle herds from sheep! Sheep are subject to a prion disease called scrapie, and the disease is thought to have passed from sheep to cows through protein-supplemented feed pellets containing ground-up sheep brains. (59)
|
In March 1996, an outbreak of [MAD COW DISEASE] in Britain, with over 80,000 cows infected, created widespread concern. BSE, a degeneration of the brain caused by prions, appears to have entered the British cattle herds from sheep! Sheep are subject to a prion disease called scrapie, and the disease is thought to have passed from sheep to cows through protein-supplemented feed pellets containing ground-up sheep brains. (59)
|
|
|
|
The passage of prions from one species to another has a scary consequence: prions can also pass from ____ to ______! At least 148 British consumers of BSE-infected beef have subsequently died of vCJD. Tissue from the brains of the dead Britons and from BSE cows induce the same brain lesions in mice, while classic CJD produces quite different lesions – clearly the form of vCJD that killed them was caused by the same agent that caused BSE. (59)
|
The passage of prions from one species to another has a scary consequence: prions can also pass from [COWS] to [PEOPLE]! At least 148 British consumers of BSE-infected beef have subsequently died of vCJD. Tissue from the brains of the dead Britons and from BSE cows induce the same brain lesions in mice, while classic CJD produces quite different lesions – clearly the form of vCJD that killed them was caused by the same agent that caused BSE. (59)
|
|
|
|
Prions and Mad Cow Disease – Can Humans Catch Mad Cow Disease by Eating Infected Meat? – The occasional appearance of BSE-infected cows in ________ and ______ ______ herds has led to heightened scrutiny of commercial beef in the United States in an attempt to eliminate any possibility of BSE contamination. (59)
|
Prions and Mad Cow Disease – Can Humans Catch Mad Cow Disease by Eating Infected Meat? – The occasional appearance of BSE-infected cows in [CANADIAN] and [UNITED STATES] herds has led to heightened scrutiny of commercial beef in the United States in an attempt to eliminate any possibility of BSE contamination. (59)
|
|
|
|
Very long polymers called nucleic acids serve as the ___________ storage devices of cells, just as DVDs or hard drives store the information that computers use. (60)
|
Very long polymers called nucleic acids serve as the [INFORMATION] storage devices of cells, just as DVDs or hard drives store the information that computers use. (60)
|
|
|
|
Nucleic acids are long ________ of repeating subunits called nucleotides. (60)
|
Nucleic acids are long [POLYMERS] of repeating subunits called nucleotides. (60)
|
|
|
|
Each __________ is a complex organic molecule composed of three parts shown in figure 3.9a: a five-carbon sugar (in blue), a phosphate group (in yellow, PO4), and an organic nitrogen-containing base (in orange). In the formation of a nucleic acid, the individual sugars with their attached nitrogenous bases are linked in a line by the phosphate groups in very long ______________ chains (shown to the right). (60)
|
Each [NUCLEOTIDE] is a complex organic molecule composed of three parts shown in figure 3.9a: a five-carbon sugar (in blue), a phosphate group (in yellow, PO4), and an organic nitrogen-containing base (in orange). In the formation of a nucleic acid, the individual sugars with their attached nitrogenous bases are linked in a line by the phosphate groups in very long [POLYNUCLEOTIDE] chains (shown to the right). (60)
|
|
|
|
How does the long, chainlike structure of a _______ ____ permit it to store the information necessary to specify what a human being is like? If _______ _____ were simply a monotonous repeating polymer, it could not encode the message of life. Imagine trying to write a story using only the letter E and no spaces or punctuation. All you could ever say is “EEEEEEEE….” You need more than one letter to communicate – the English alphabet uses 26 letters. (60)
|
How does the long, chainlike structure of a [NUCLEIC ACID] permit it to store the information necessary to specify what a human being is like? If [NUCLEIC ACIDS] were simply a monotonous repeating polymer, it could not encode the message of life. Imagine trying to write a story using only the letter E and no spaces or punctuation. All you could ever say is “EEEEEEEE….” You need more than one letter to communicate – the English alphabet uses 26 letters. (60)
|
|
|
|
_______ _____ can encode information because they contain more than one kind of nucleotide. There are five different kinds of nucleotides: two larger ones that contain the nitrogenous bases _______ and _______ (shown in the top row of figure 3.9b), and three smaller ones that contain the nitrogenous bases ________, _______, and ______ (in the bottom row). (60)
|
[NUCLEIC ACIDS] can encode information because they contain more than one kind of nucleotide. There are five different kinds of nucleotides: two larger ones that contain the nitrogenous bases [ADENINE] and [GUANINE] (shown in the top row of figure 3.9b), and three smaller ones that contain the nitrogenous bases [CYTOSINE], [THYMINE], and [URACIL] (in the bottom row). (60)
|
|
|
|
Nucleic acids encode information by varying the ________ of the nucleotide at each position in the polymer. (60)
|
Nucleic acids encode information by varying the [IDENTITY] of the nucleotide at each position in the polymer. (60)
|
|
|
|
Nucleic acids come in two varieties, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both ________ of nucleotides with some differences. (60)
|
Nucleic acids come in two varieties, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both [POLYMERS] of nucleotides with some differences. (60)
|
|
|
|
RNA is similar to DNA but with two major chemical differences. First, RNA molecules contain the sugar ______, in which the 2’carbon (this is the carbon labeled 2’ in figure 3.9a) is bonded to a hydroxyl group (-OH). In DNA, this hydroxyl group is replaced with a ________ ____. Second, RNA molecules do not contain the _______ nucleotide; they contain ______ instead. Structurally, RNA is also different. RNA is a long, single strand of nucleotides and is used by cells in making proteins using genetic instructions encoded within DNA. (60)
|
RNA is similar to DNA but with two major chemical differences. First, RNA molecules contain the sugar [RIBOSE], in which the 2’carbon (this is the carbon labeled 2’ in figure 3.9a) is bonded to a hydroxyl group (-OH). In DNA, this hydroxyl group is replaced with a [HYDROGEN ATOM]. Second, RNA molecules do not contain the [THYMINE] nucleotide; they contain [URACIL] instead. Structurally, RNA is also different. RNA is a long, single strand of nucleotides and is used by cells in making proteins using genetic instructions encoded within DNA. (60)
|
|
|
|
The ________ of nucleotides in DNA determines the order of amino acids in the _______ structure of the protein. (60)
|
The [SEQUENCE] of nucleotides in DNA determines the order of amino acids in the [PRIMARY] structure of the protein. (60)
|
|
|
|
DNA consists of two polynucleotide chains would around each other in a ______ _____, like strands of a pearl necklace twisted together. You can see this difference in structure by comparing the blue double-stranded DNA molecule in figure 3.10 with the green single-stranded RNA molecule. (61)
|
DNA consists of two polynucleotide chains would around each other in a [DOUBLE HELIX], like strands of a pearl necklace twisted together. You can see this difference in structure by comparing the blue double-stranded DNA molecule in figure 3.10 with the green single-stranded RNA molecule. (61)
|
|
|
|
Figure 3.9 – The structure of a nucleotide. (a) Nucleotides are composed of three parts: a ____-______ _____, a _________ _____, and an organic ___________ ____. (b) The ___________ ____ can be one of five. (60)
|
Figure 3.9 – The structure of a nucleotide. (a) Nucleotides are composed of three parts: a [FIVE-CARBON SUGAR], a [PHOSPHATE GROUP], and an organic [NITROGENOUS BASE]. (b) The [NITROGENOUS BASE] can be one of five. (60)
|
|
|
|
Figure 3.10 – How DNA structure differs from RNA. (a) DNA contains two ______________ strands wrapped around each other, while (b) RNA is ______-________. (60)
|
Figure 3.10 – How DNA structure differs from RNA. (a) DNA contains two [POLYNUCLEOTIDE] strands wrapped around each other, while (b) RNA is [SINGLE-STRANDED]. (60)
|
|
|
|
Why is DNA a double helix? When scientists looked carefully at the structure of the DNA double helix, they found that the bases of each chain point ______ toward the other (like the DNA strands shown in figure 3.11). The bases of the two chains are linked in the middle of the molecule by ________ _____ (the dotted lines between the two strands), like two columns of people holding hands across. (61)
|
Why is DNA a double helix? When scientists looked carefully at the structure of the DNA double helix, they found that the bases of each chain point [INWARD] toward the other (like the DNA strands shown in figure 3.11). The bases of the two chains are linked in the middle of the molecule by [HYDROGEN BONDS] (the dotted lines between the two strands), like two columns of people holding hands across. (61)
|
|
|
|
The key to understanding why DNA is a ______ _____ is revealed by looking at the bases: only two base pairs are possible. Because the distance between the two strands is __________, this suggests that two big bases cannot ____ together – the combination is simply too bulky to fit; similarly, two little ones cannot pair, as they pinch the helix inward too much. (61)
|
The key to understanding why DNA is a [DOUBLE HELIX] is revealed by looking at the bases: only two base pairs are possible. Because the distance between the two strands is [CONSISTENT], this suggests that two big bases cannot [PAIR] together – the combination is simply too bulky to fit; similarly, two little ones cannot pair, as they pinch the helix inward too much. (61)
|
|
|
|
To form a double helix, it is necessary to pair a ___ ____ with a ______ one. In every DNA double helix, adenine (A) pairs with thymine (T) and guanine (G) pairs with cytosine (C). The reason A doesn’t pair with C and G doesn’t pair with T is that these base pairs cannot form proper ________ _____ – the electron-sharing atoms are not aligned with each other. (61)
|
To form a double helix, it is necessary to pair a [BIG BASE] with a [LITTLE] one. In every DNA double helix, adenine (A) pairs with thymine (T) and guanine (G) pairs with cytosine (C). The reason A doesn’t pair with C and G doesn’t pair with T is that these base pairs cannot form proper [HYDROGEN BONDS] – the electron-sharing atoms are not aligned with each other. (61) |
|
|
|
The simple A-T, G-C base pairs within the DNA double helix allow the cell to ____ the information in a very simple way. It just unzips the helix and adds the complementary bases to each new strand! That is the great advantage of a double helix – it actually contains two copies of the information, one the mirror image of the other. If the sequence of one chain is ATTGCAT, the sequence of its partner in the double helix must be TAACGTA. The fidelity with which hereditary information is passed from one generation to the next is a direct result of this simple double-entry bookkeeping. (61)
|
The simple A-T, G-C base pairs within the DNA double helix allow the cell to [COPY] the information in a very simple way. It just unzips the helix and adds the complementary bases to each new strand! That is the great advantage of a double helix – it actually contains two copies of the information, one the mirror image of the other. If the sequence of one chain is ATTGCAT, the sequence of its partner in the double helix must be TAACGTA. The fidelity with which hereditary information is passed from one generation to the next is a direct result of this simple double-entry bookkeeping. (61)
|
|
|
|
Figure 3.11 – The DNA double helix. The DNA molecule is composed of two ______________ chains twisted together to form a double helix. The two chains of the double helix are joined by ________ _____ between the A-T and G-C base pairs. The section of DNA on the upper right is a space-filling model of DNA, where atoms are indicated by colored balls. (61)
|
Figure 3.11 – The DNA double helix. The DNA molecule is composed of two [POLYNUCLEOTIDE] chains twisted together to form a double helix. The two chains of the double helix are joined by [HYDROGEN BONDS] between the A-T and G-C base pairs. The section of DNA on the upper right is a space-filling model of DNA, where atoms are indicated by colored balls. (61)
|
|
|
|
Key Learning Outcome 3.3 – _______ acids like DNA are composed of long chains of nucleotides. The sequence of the nucleotides specifies the _____ acid sequence of proteins. (61)
|
Key Learning Outcome 3.3 – [NUCLEIC] acids like DNA are composed of long chains of nucleotides. The sequence of the nucleotides specifies the [AMINO] acid sequence of proteins. (61)
|
|
|
|
Polymers called _____________ make up the structural framework of cells and play a critical role in ______ storage. (63)
|
[POLYMERS] called carbohydrates make up the structural framework of cells and play a critical role in [ENERGY] storage. (63)
|
|
|
|
A carbohydrate is any molecule that contains ______, ________, and ______ in the ratio 1:2:1. (63)
|
A carbohydrate is any molecule that contains carbon, hydrogen, and oxygen in the ratio 1:2:1. (63)
|
|
|
|
Because they contain many carbon-hydrogen (C-H) bonds, carbohydrates are well-suited for ______ _______. Such C-H bonds are the ones most often broken by organisms to obtain ______. Table 3.2 on the following page shows some examples of carbohydrates. (63)
|
Because they contain many carbon-hydrogen (C-H) bonds, carbohydrates are well-suited for [ENERGY STORAGE]. Such C-H bonds are the ones most often broken by organisms to obtain [Energy]. Table 3.2 on the following page shows some examples of carbohydrates. (63)
|
|
|
|
The simplest carbohydrates are the simple sugars or _______________ (from the Greek monos, single, and saccharin, sweet). These molecules consist of one subunit. For example, glucose, the sugar that carries energy to the cells of your body, is made of chain of six carbons and has the chemical formula C6H12O6 (figure 3.12). When placed in water, the chain folds into the ring structure shown on the lower right of the figure. The individual atoms are depicted in the “3-D” space-filling model you see in the lower left of the figure. (63)
|
The simplest carbohydrates are the simple sugars or [MONOSACCHARIDES] (from the Greek monos, single, and saccharin, sweet). These molecules consist of one subunit. For example, glucose, the sugar that carries energy to the cells of your body, is made of chain of six carbons and has the chemical formula C6H12O6 (figure 3.12). When placed in water, the chain folds into the ring structure shown on the lower right of the figure. The individual atoms are depicted in the “3-D” space-filling model you see in the lower left of the figure. (63)
|
|
|
|
Another type of simple carbohydrate is a ____________, which forms when two monosaccharides link together through a dehydration reaction. In figure 3.13, you can see how the ____________ sucrose (table sugar) is made by linking two six-carbon sugars together, a glucose (orange) and a fructose (green). (63)
|
Another type of simple carbohydrate is a [DISACCHARIDE], which forms when two monosaccharides link together through a dehydration reaction. In figure 3.13, you can see how the [DISACCHARIDE] sucrose (table sugar) is made by linking two six-carbon sugars together, a glucose (orange) and a fructose (green). (63)
|
|
|
|
Organisms store their metabolic energy by __________ sugars, which are soluble, into insoluble forms that can be deposited in specific storage areas in the body. This trick is achieved by linking the sugars together into long polymer chains called _______________. (63)
|
Organisms store their metabolic energy by [CONVERTING] sugars, which are soluble, into insoluble forms that can be deposited in specific storage areas in the body. This trick is achieved by linking the sugars together into long polymer chains called [POLYSACCHARIDES]. (63)
|
|
|
|
Plants and animals store energy in polysaccharides formed from glucose. The glucose polysaccharide that plants use to store energy is called ______. In animals, energy is stored in ________, an insoluble macromolecule formed of long glucose polysaccharides that are highly branched. (63)
|
Plants and animals store energy in polysaccharides formed from glucose. The glucose polysaccharide that plants use to store energy is called [STARCH]. In animals, energy is stored in [GLYCOGEN], an insoluble macromolecule formed of long glucose polysaccharides that are highly branched. (63)
|
|
|
|
Plants and animals also use _______ chains as building materials, linking the subunits together in different orientations not recognized by most enzymes. These structural polysaccharides are ______, in animals, and _________, in plants. (63)
|
Plants and animals also use [GLUCOSE] chains as building materials, linking the subunits together in different orientations not recognized by most enzymes. These structural polysaccharides are [CHITIN], in animals, and [CELLULOSE], in plants. (63)
|
|
|
|
The cellulose deposited in the ____ _____ of plant cells, like the cellulose strand shown in figure 3.14, cannot be ________ by humans and makes up the fiber in our diets. (63)
|
The cellulose deposited in the [CELL WALLS] of plant cells, like the cellulose strand shown in figure 3.14, cannot be [DIGESTED] by humans and makes up the fiber in our diets. (63)
|
|
|
|
Key Learning Outcome 3.4 – _____________ are molecules made of C, H, and O atoms. As sugars they store energy in C-H bonds, and as long polysaccharide chains they can provide __________ support. (63)
|
Key Learning Outcome 3.4 – [CARBOHYDRATES] are molecules made of C, H, and O atoms. As sugars they store energy in C-H bonds, and as long polysaccharide chains they can provide [STRUCTURAL] support. (63)
|
|
|
|
Figure 3.12 – The structure of glucose. Glucose is a ______________ and consists of linear six-carbon molecule that forms a ring when added to water. This illustration shows three ways glucose can be represented diagrammatically. (63)
|
Figure 3.12 – The structure of glucose. Glucose is a [MONOSACCHARIDE] and consists of linear six-carbon molecule that forms a ring when added to water. This illustration shows three ways glucose can be represented diagrammatically. (63)
|
|
|
|
Figure 3.13 – Formation of sucrose. The ____________ sucrose is formed from glucose and fructose in a dehydration reaction. (63)
|
Figure 3.13 – Formation of sucrose. The [DISACCHARIDE] sucrose is formed from glucose and fructose in a dehydration reaction. (63)
|
|
|
|
Figure 3.14 – A polysaccharide: cellulose. The polysaccharide cellulose is found in the cell walls of _____ _____ and is composed of glucose subunits. (63)
|
Figure 3.14 – A polysaccharide: cellulose. The polysaccharide cellulose is found in the cell walls of [PLANT CELLS] and is composed of glucose subunits. (63)
|
|
|
|
For ____-term energy storage, organisms usually convert glucose into ____, another kind of storage molecule that contains more energy-rich C-H bonds than carbohydrates. (65)
|
For [LONG]-term energy storage, organisms usually convert glucose into [FATS], another kind of storage molecule that contains more energy-rich C-H bonds than carbohydrates. (65)
|
|
|
|
Fats and all other biological molecules that are not soluble in _____ but are soluble in oil are called ______. (65)
|
Fats and all other biological molecules that are not soluble in [WATER] but are soluble in oil are called [LIPIDS]. (65)
|
|
|
|
Lipids are _________ in water not because they are long chains like starches but rather because they are nonpolar. In water, fat molecules cluster together because they cannot form ________ _____ with water molecules. This is why oil forms into a layer on top of water when the two substances are mixed. (65)
|
Lipids are [INSOLUBLE] in water not because they are long chains like starches but rather because they are nonpolar. In water, fat molecules cluster together because they cannot form [HYDROGEN BONDS] with water molecules. This is why oil forms into a layer on top of water when the two substances are mixed. (65)
|
|
|
|
Fat molecules are ______ composed of two kinds of subunits: _____ _____ (the gray boxed structures in figure 3.15a) and ________ (the orange boxed structure). (65)
|
Fat molecules are [LIPIDS] composed of two kinds of subunits: [FATTY ACIDS] (the gray boxed structures in figure 3.15a) and [GLYCEROL] (the orange boxed structure). (65) |
|
|
|
A _____ ____ is a long chain of carbon and hydrogen atoms (called a hydrocarbon) ending in a carboxyl (-COOH) group. The three carbons of glycerol form the backbone to which three _____ _____ are attaching the dehydration reaction that forms the fat molecule. That is why the carboxyl groups of the _____ _____ in figure 3.15a are not apparent; they are involved in bonds with glycerol. Because it contains three _____ _____, the resulting fat molecule is sometimes called a triacylglycerol, or triglyceride. (65)
|
A [FATTY ACID] is a long chain of carbon and hydrogen atoms (called a hydrocarbon) ending in a carboxyl (-COOH) group. The three carbons of glycerol form the backbone to which three [FATTY ACIDS] are attaching the dehydration reaction that forms the fat molecule. That is why the carboxyl groups of the [FATTY ACIDS] in figure 3.15a are not apparent; they are involved in bonds with glycerol. Because it contains three [FATTY ACIDS], the resulting fat molecule is sometimes called a triacylglycerol, or triglyceride. (65)
|
|
|
|
Fatty acids with all internal carbon atoms forming covalent bonds with two hydrogen atoms contain the _______ number of hydrogen atoms. Fats composed of these fatty acids are said to be _________ (figure 3.15b). (65)
|
Fatty acids with all internal carbon atoms forming covalent bonds with two hydrogen atoms contain the [MAXIMUM] number of hydrogen atoms. Fats composed of these fatty acids are said to be [SATURATED] (figure 3.15b). (65)
|
|
|
|
Saturated fats are _____ at room temperature. On the other hand, fats composed of fatty acids that have double bonds between one or more pairs of carbon atoms contain fewer than the maximum number of hydrogen atoms and are called ___________ (figure 3.15c). The double bonds create kinks in the fatty acid tails, which usually makes the unsaturated fats ______ at room temperature. (65)
|
Saturated fats are [SOLID] at room temperature. On the other hand, fats composed of fatty acids that have double bonds between one or more pairs of carbon atoms contain fewer than the maximum number of hydrogen atoms and are called [UNSATURATED] (figure 3.15c). The double bonds create kinks in the fatty acid tails, which usually makes the unsaturated fats [LIQUID] at room temperature. (65)
|
|
|
|
Many _____ fats are unsaturated and occur as oils. ______ fats, in contrast, are often saturated and occur as hard fats. (65)
|
Many [Plant] fats are unsaturated and occur as oils. [Animal] fats, in contrast, are often saturated and occur as hard fats. (65)
|
|
|
|
In some cases, unsaturated fats in food products may be artificially ____________ (industrial addition of hydrogens) to make them more saturated, extending the shelf life of these products. In some cases, the _____________ creates trans fats, a type of unsaturated fat in which some of the double bonds are less kinked than those in naturally occurring unsaturated fats. (65)
|
In some cases, unsaturated fats in food products may be artificially [HYDROGENATED] (industrial addition of hydrogens) to make them more saturated, extending the shelf life of these products. In some cases, the [HYDROGENATION] creates trans fats, a type of unsaturated fat in which some of the double bonds are less kinked than those in naturally occurring unsaturated fats. (65)
|
|
|
|
Eating _____ fats and _________ fats may increase the risk of heart disease. (65)
|
Eating [TRANS] fats and [SATURATED] fats may increase the risk of heart disease. (65)
|
|
|
|
Organisms also contain other types of ______ that play many roles in cells in addition to ______ storage. (65)
|
Organisms also contain other types of [LIPIDS] that play many roles in cells in addition to [ENERGY] storage. (65)
|
|
|
|
The male and female sex hormones testosterone and estradiol are ______ called ________. (65)
|
The male and female sex hormones testosterone and estradiol are [LIPIDS] called [STEROIDS]. (65)
|
|
|
|
Unlike the structure of fats shown in figure 3.15, the structure of steroids consists of multiple ____ structures and looks somewhat like a section of chicken wire. (65)
|
Unlike the structure of fats shown in figure 3.15, the structure of steroids consists of multiple [RING] structures and looks somewhat like a section of chicken wire. (65)
|
|
|
|
Other important biological lipids include _____________, ___________ (also a steroid), ______, _____, and ________, such as the chlorophyll that makes plants green and the retinal that your eyes use to detect light (see figure 3.16). (65)
|
Other important biological lipids include [PHOSPHOLIPIDS], [CHOLESTEROL] (also a steroid), [RUBBER], [WAXES], and [PIGMENTS], such as the chlorophyll that makes plants green and the retinal that your eyes use to detect light (see figure 3.16). (65)
|
|
|
|
Figure 3.15 – Saturated and unsaturated fats. (a) Fat molecules each contain a three-carbon glycerol to which is attached three fatty acid tails. (b) Most ______ fats are “saturated” (every carbon atom carries the maximum load of hydrogens). Their fatty acid chains fit closely together, and these triacylglycerols form immobile arrays called hard fats. (c) Most _____ fats are unsaturated, which prevents close association between triacylglycerols and produces oils. (65)
|
Figure 3.15 – Saturated and unsaturated fats. (a) Fat molecules each contain a three-carbon glycerol to which is attached three fatty acid tails. (b) Most [ANIMAL] fats are “saturated” (every carbon atom carries the maximum load of hydrogens). Their fatty acid chains fit closely together, and these triacylglycerols form immobile arrays called hard fats. (c) Most [PLANT] fats are unsaturated, which prevents close association between triacylglycerols and produces oils. (65)
|
|
|
|
If you look at figure 3.17, you will see two types of lipids that play key roles in the membranes that encase the cells of your body: ____________ molecules and ___________. (67)
|
If you look at figure 3.17, you will see two types of lipids that play key roles in the membranes that encase the cells of your body: [PHOSPHOLIPID] molecules and [CHOLESTEROL]. (67)
|
|
|
|
_____________ are modified triacylglycerol molecules in which one of the carbons in the glycerol backbone bonds to a phosphate group rather than to a third fatty acid (compare figure 3.15a and 3.16a). This gives the molecule an overall shape of a polar ____ and two nonpolar _____. (67)
|
[PHOSPHOLIPIDS] are modified triacylglycerol molecules in which one of the carbons in the glycerol backbone bonds to a phosphate group rather than to a third fatty acid (compare figure 3.15a and 3.16a). This gives the molecule an overall shape of a polar [HEAD] and two nonpolar [TAILS]. (67)
|
|
|
|
In water, the ________ ends of the phospholipids group together through hydrophobic interactions, leaving the _____ heads to interact with water molecules. These interactions result in the formation of two layers of molecules with the ________ tails pointed inside – a lipid bilayer. All biological membranes, those that surround the cell and those found inside the cell, have this arrangement. (67)
|
In water, the [NONPOLAR] ends of the phospholipids group together through hydrophobic interactions, leaving the [POLAR] heads to interact with water molecules. These interactions result in the formation of two layers of molecules with the [NONPOLAR] tails pointed inside – a lipid bilayer. All biological membranes, those that surround the cell and those found inside the cell, have this arrangement. (67)
|
|
|
|
Most animal cell membranes also contain the steroid ___________, a lipid composed of four carbon rings (see figure 3.16b). (67)
|
Most animal cell membranes also contain the steroid [CHOLESTEROL], a lipid composed of four carbon rings (see figure 3.16b). (67)
|
|
|
|
Cholesterol helps membranes stay ________. However, excess saturated fat intake can cause plugs of cholesterol to form in the _____ _______, which may lead to blockage, high blood pressure, stroke, or heart attack. (67)
|
Cholesterol helps membranes stay [FLEXIBLE]. However, excess saturated fat intake can cause plugs of cholesterol to form in the [BLOOD VESSELS], which may lead to blockage, high blood pressure, stroke, or heart attack. (67)
|
|
|
|
Key Learning Outcome 3.5 – Lipids are not _____-_______. (67)
|
Key Learning Outcome 3.5 – Lipids are not [WATER-SOLUBLE]. (67)
|
|
|
|
Fats contain chains of fatty acid ________ that store energy. (67)
|
Fats contain chains of fatty acid [SUBUNITS] that store energy. (67)
|
|
|
|
Other lipids include _____________, ________, and ______. (67)
|
Other lipids include [PHOSPHOLIPIDS], [STEROIDS], and [RUBBER]. (67)
|
|
|
|
Figure 3.16 – Types of lipid molecules. (a) A ____________ is similar to a fat molecule with the exception that one of the fatty acid tails is replaced with a polar group linked to the glycerol by a phosphate group. (67)
|
Figure 3.16 – Types of lipid molecules. (a) A [PHOSPHOLIPID] is similar to a fat molecule with the exception that one of the fatty acid tails is replaced with a polar group linked to the glycerol by a phosphate group. (67) |
|
|
|
Figure 3.16 – Types of lipid molecules. (b) ________ like cholesterol are lipids with complex ring structures. (67)
|
Figure 3.16 – Types of lipid molecules. (b) [STEROIDS] like cholesterol are lipids with complex ring structures. (67)
|
|
|
|
Figure 3.16 – Types of lipid molecules. (c) _______ ______ is a linear polymer of a five-carbon unit called isoprene (two isoprene units are shown here; one unit has its carbon in green and the other in red). ______, made from latex harvested from rubber trees, is used in many products such as car tires.
|
Figure 3.16 – Types of lipid molecules. (c) [NATURAL RUBBER] is a linear polymer of a five-carbon unit called isoprene (two isoprene units are shown here; one unit has its carbon in green and the other in red). [RUBBER], made from latex harvested from rubber trees, is used in many products such as car tires.
|
|
|
|
Figure 3.16 – Types of lipid molecules. (d) ___________ a has a multiringed area and a long hydrocarbon tail. Chlorophyll is the primary pigment used in photosynthesis, and it is what makes leaves green. (67)
|
Figure 3.16 – Types of lipid molecules. (d) [CHLOROPHYLL] a has a multiringed area and a long hydrocarbon tail. Chlorophyll is the primary pigment used in photosynthesis, and it is what makes leaves green. (67)
|
|
|
|
Figure 3.17 – Lipids are a key component of biological _________. (67)
|
Figure 3.17 – Lipids are a key component of biological [MEMBRANES]. (67)
|
|
|
|
Lipids are one of the most ______ molecules in the human body, because the membranes of all the body’s 10 trillion cells are composed largely of lipids called _____________. (67)
|
Lipids are one of the most [COMMON] molecules in the human body, because the membranes of all the body’s 10 trillion cells are composed largely of lipids called [PHOSPHOLIPIDS]. (67)
|
|
|
|
Membranes also contain ___________, another type of lipid. (67)
|
Membranes also contain [CHOLESTEROL], another type of lipid. (67)
|
|
|
|
3.1.1 Living organisms produce organic macromolecules, which are large ______-based molecules. The chemical properties of these molecules are due to unique functional groups that are attached to the ______ core. (69)
|
3.1.1 Living organisms produce organic macromolecules, which are large [CARBON]-based molecules. The chemical properties of these molecules are due to unique functional groups that are attached to the [CARBON] core. (69)
|
|
|
|
3.1.2a Large molecules called ______________ are formed by dehydration reactions (figure 3.3a), in which molecular subunits called ________ are linked together through covalent bonding. The reaction is called a dehydration reaction because a water molecule is a _______ of the reaction. (69)
|
3.1.2a Large molecules called [MACROMOLECULES] are formed by dehydration reactions (figure 3.3a), in which molecular subunits called [MONOMERS] are linked together through covalent bonding. The reaction is called a dehydration reaction because a water molecule is a [PRODUCT] of the reaction. (69)
|
|
|
|
3.1.2b The breakdown of a macromolecule involves a __________ reaction, where a water molecule is broken down into H+ and OH-. These ____ disrupt the covalent bond that holds two monomers together, causing the bond to break. (69)
|
3.1.2b The breakdown of a macromolecule involves a [HYDROLYSIS] reaction, where a water molecule is broken down into H+ and OH-. These [IONS] disrupt the covalent bond that holds two monomers together, causing the bond to break. (69)
|
|
|
|
3.1.2c _____ _____ are the subunits that link together to form polypeptides. (69)
|
3.1.2c [AMINO ACIDS] are the subunits that link together to form polypeptides. (69)
|
|
|
|
__________ ________ link together to form nucleic acids. (69)
|
[NUCLEOTIDE MONOMERS] link together to form nucleic acids. (69)
|
|
|
|
______________ ________ link together to form carbohydrates. (69)
|
[MONOSACCHARIDE MONOMERS] link together to form carbohydrates. (69)
|
|
|
|
_____ _____ are the monomers that link together to form a type of lipid called fats. (69)
|
[FATTY ACIDS] are the monomers that link together to form a type of lipid called fats. (69)
|
|
|
|
3.2.1a ________ are macromolecules that carry out many functions in the cell. They are produced by the linking together of _____ ____ subunits that form a chain called a polypeptide. (69)
|
3.2.1a [PROTEINS] are macromolecules that carry out many functions in the cell. They are produced by the linking together of [AMINO ACID] subunits that form a chain called a polypeptide. (69)
|
|
|
|
3.2.1b There are 20 different _____ _____ found in proteins. (69)
|
3.2.1b There are 20 different [AMINO ACIDS] found in proteins. (69)
|
|
|
|
All amino acids have the same basic core _________. They differ in the type of __________ _____ attached to the core. (69)
|
All amino acids have the same basic core [STRUCTURE]. They differ in the type of [FUNCTIONAL GROUP] attached to the core. (69)
|
|
|
|
The functional groups are referred to as _ ______ and give the amino acid ______ chemical properties. (69)
|
The functional groups are referred to as [R GROUPS] and give the amino acid [UNIQUE] chemical properties. (69)
|
|
|
|
3.2.2 Amino acids are linked together with covalent bonds referred to as _______ _____. (69)
|
3.2.2 Amino acids are linked together with covalent bonds referred to as [PEPTIDE BONDS]. (69)
|
|
|
|
3.2.3 The sequence of amino acids within the polypeptide is the _______ structure of the protein. (69)
|
3.2.3 The sequence of amino acids within the polypeptide is the [PRIMARY] structure of the protein. (69)
|
|
|
|
The chain of amino acids can twist into a _________ structure, where hydrogen bonding holds portions of the polypeptide in a ______ shape, called an α-helix, or in ______ called β-pleated ______. (69)
|
The chain of amino acids can twist into a [SECONDARY] structure, where hydrogen bonding holds portions of the polypeptide in a [COILED] shape, called an α-helix, or in [SHEETS] called β-pleated [SHEETS]. (69) |
|
|
|
Further bending and folding of the polypeptide results in its ________ structure. (69)
|
Further bending and folding of the polypeptide results in its [TERTIARY] structure. (69)
|
|
|
|
When two or more polypeptides are present in a protein, the interaction of these polypeptide subunits is its __________ structure. (69)
|
When two or more polypeptides are present in a protein, the interaction of these polypeptide subunits is its [QUATERNARY] structure. (69)
|
|
|
|
3.2.4 Changes in environmental conditions that disrupt ________ bonding can cause a protein to unfold, a process called ____________. (69)
|
3.2.4 Changes in environmental conditions that disrupt [HYDROGEN] bonding can cause a protein to unfold, a process called [DENATURATION]. (69)
|
|
|
|
3.2.5 ________ proteins (page 57) cannot function if they are denatured. The _________ of the protein determines its function. (69)
|
3.2.5 [GLOBULAR] proteins (page 57) cannot function if they are denatured. The [STRUCTURE] of the protein determines its function. (69)
|
|
|
|
3.2.6 _________ proteins aid in the folding of the amino acid chain into the correct _____. (69)
|
3.2.6 [CHAPERONE] proteins aid in the folding of the amino acid chain into the correct [SHAPE]. (69)
|
|
|
|
3.2.7 Some diseases may be caused by misfolded, and therefore _____________, proteins. (69)
|
3.2.7 Some diseases may be caused by misfolded, and therefore [NONFUNCTIONAL], proteins. (69)
|
|
|
|
3.3.1 Nucleic acids, such as DNA and RNA, are long chains of nucleotides. Nucleotides contain three parts: a ____-______ _____, a _________ _____, and a ___________ ____. (69)
|
3.3.1 Nucleic acids, such as DNA and RNA, are long chains of nucleotides. Nucleotides contain three parts: a [FIVE-CARBON SUGAR], a [PHOSPHATE GROUP], and a [NITROGENOUS BASE]. (69)
|
|
|
|
DNA and RNA function as information storage molecules in the cell, storing the information needed to build ________. The information is stored as different _________ of nucleotides. (69)
|
DNA and RNA function as information storage molecules in the cell, storing the information needed to build [PROTEINS]. The information is stored as different [SEQUENCES] of nucleotides. (69)
|
|
|
|
3.3.2a DNA and RNA differ chemically in that the sugar found in DNA is ___________ and in RNA is ______. (69)
|
3.3.2a DNA and RNA differ chemically in that the sugar found in DNA is [DEOXYRIBOSE] and in RNA is [RIBOSE]. (69)
|
|
|
|
The nitrogenous bases in DNA are ________, _______, _______, and _______, and the same are found in RNA except for _______, which is substituted with ______. (69)
|
The nitrogenous bases in DNA are [CYTOSINE], [ADENINE], [GUANINE], and [THYMINE], and the same are found in RNA except for [THYMINE], which is substituted with [URACIL]. (69)
|
|
|
|
3.3.2b DNA and RNA also differ ____________. DNA contains two strands of nucleotides wound around each other, called a ______ _____. RNA, as shown in figure 3.10, is a ______ strand of nucleotides. (69)
|
3.3.2b DNA and RNA also differ [STRUCTURALLY]. DNA contains two strands of nucleotides wound around each other, called a [DOUBLE HELIX]. RNA, as shown in figure 3.10, is a [SINGLE] strand of nucleotides. (69)
|
|
|
|
3.3.3 The two nucleotide strands of the DNA double helix are held together through highly directional ________ bonding between ___________ bases: adenine (A) pairs with thymine (T) and cytosine (C) pairs with guanine (G). (69)
|
3.3.3 The two nucleotide strands of the DNA double helix are held together through highly directional [HYDROGEN] bonding between [NITROGENOUS] bases: adenine (A) pairs with thymine (T) and cytosine (C) pairs with guanine (G). (69)
|
|
|
|
3.4.1a Carbohydrates, also referred to as sugars, are macromolecules that serve two primary functions in the cell: __________ framework and ______ storage. (69)
|
3.4.1a Carbohydrates, also referred to as sugars, are macromolecules that serve two primary functions in the cell: [STRUCTURAL] framework and [ENERGY] storage. (69)
|
|
|
|
3.4.1b Carbohydrates that consist of only one or two monomers are called ______ ______ (figure 3.12). Carbohydrates that consist of long chains of monomers are called _______ _____________ or polysaccharides. (69)
|
3.4.1b Carbohydrates that consist of only one or two monomers are called [SIMPLE SUGARS] (figure 3.12). Carbohydrates that consist of long chains of monomers are called [COMPLEX CARBOHYDRATES] or polysaccharides. (69)
|
|
|
|
3.4.2 Polysaccharides such as starch and glycogen provide a means of _______ ______ in the cell. Carbohydrates such as cellulose and chitin cannot be digested by most organisms, and serve to provide __________ _________. (69)
|
3.4.2 Polysaccharides such as starch and glycogen provide a means of [STORING ENERGY] in the cell. Carbohydrates such as cellulose and chitin cannot be digested by most organisms, and serve to provide [STRUCTURAL INTEGRITY]. (69)
|
|
|
|
3.5.1 ______ are large nonpolar molecules that are insoluble in water. (69)
|
3.5.1 [LIPIDS] are large nonpolar molecules that are insoluble in water. (69)
|
|
|
|
Fats that function in ____-term ______ storage include saturated fats (figure 3.15) and unsaturated fats. (69)
|
Fats that function in [LONG]-term [ENERGY] storage include saturated fats (figure 3.15) and unsaturated fats. (69)
|
|
|
|
_________ fats are solid at room temperature and are found in _______, while ___________ fats are liquid (oils) at room temperature and found in ______. (69)
|
[SATURATED] fats are solid at room temperature and are found in [ANIMALS], while [UNSATURATED] fats are liquid (oils) at room temperature and found in [PLANTS]. (69)
|
|
|
|
3.5.2 Other ______ include the steroids (including the sex steroids and cholesterol), rubber, and pigments such as chlorophyll. All have very different __________ and very different _________. (69)
|
3.5.2 Other [LIPIDS] include the steroids (including the sex steroids and cholesterol), rubber, and pigments such as chlorophyll. All have very different [STRUCTURES] and very different [FUNCTIONS]. (69)
|
|
|
|
All cells are encased in a ______ ________, called the lipid bilayer, that is composed of ___ layers of modified fat molecules called phospholipids, which are quite _____. (69) |
All cells are encased in a [PLASMA MEMBRANE], called the lipid bilayer, that is composed of [TWO] layers of modified fat molecules called phospholipids, which are quite [POLAR]. (69) |
|
|
|
3.1.1 The four kinds of organic macromolecules are: (70) |
3.1.1 The four kinds of organic macromolecules are: (70) |
|
|
|
3.1.2 Organic molecules are made up of monomers. Which of the following are NOT considered monomers of organic molecules? (70) |
3.1.2 Organic molecules are made up of monomers. Which of the following are NOT considered monomers of organic molecules? (70) c.Polypeptides |
|
|
|
3.2.1a Your body is filled with many types of proteins. Each type has a distinctive sequence of amino acids that determines both its specialized _______ and its specialized _______. (70) |
3.2.1a Your body is filled with many types of proteins. Each type has a distinctive sequence of amino acids that determines both its specialized _______ and its specialized _______. (70) |
|
|
|
3.2.1b A peptide bond forms: (70) |
3.2.1b A peptide bond forms: (70) |
|
|
|
3.2.2a In dehydration synthesis, water: (70) |
3.2.2a In dehydration synthesis, water: (70) |
|
|
|
3.2.2b How many molecules of water are used up in the breakdown of a polypeptide that is 14 amino acids in length? (70) |
How many molecules of water are used up in the breakdown of a polypeptide that is 14 amino acids in length? (70) |
|
|
|
3.2.3a The four general levels of protein structure do NOT include _______ structure. (70) |
3.2.3a The four general levels of protein structure do NOT include _______ structure. (70) |
|
|
|
3.2.3b Which of the following amino acids would be expected to occur in the interior of a globular protein? (70) |
3.2.3b Which of the following amino acids would be expected to occur in the interior of a globular protein? (70) |
|
|
|
3.2.4a The denaturation of a protein is typically the result of: (70) |
3.2.4a The denaturation of a protein is typically the result of: (70) |
|
|
|
3.2.4b A single-subunit protein that is denatured by heat loses its: (70) |
3.2.4b A single-subunit protein that is denatured by heat loses its: (70) |
|
|
|
3.2.5a Which of the following in NOT a function of structural proteins? (70) |
3.2.5a Which of the following in NOT a function of structural proteins? (70) |
|
|
|
3.2.5b Catalysis is: (70) |
3.2.5b Catalysis is: (70) |
|
|
|
3.2.6 If a protein’s primary structure dictates its tertiary structure and thus its function, why would it need a chaperone protein to fold correctly? (70) |
If a protein’s primary structure dictates its tertiary structure and thus its function, why would it need a chaperone protein to fold correctly? (70) |
|
|
|
3.2.7 A prion is a(n): (70) |
3.2.7 A prion is a(n): (70) |
|
|
|
3.3.1a Nucleic acids: (70) |
3.3.1a Nucleic acids: (70) |
|
|
|
3.3.1b A nucleotide is composed of all of the following except: (70) |
3.3.1b A nucleotide is composed of all of the following except: (70) |
|
|
|
3.3.2a Which carbohydrate is part of a molecule of RNA? (70) |
3.3.2a Which carbohydrate is part of a molecule of RNA? (70) |
|
|
|
3.3.2b DNA exists in cells as a double-stranded duplex molecule, whereas RNA, which is composed of very similar nucleotides linked together in the same way, does not form a double-stranded duplex in cells. Why not? If you were to place complementary single-strands of RNA in a test tube, would they spontaneously form duplex molecules? (70) |
DNA exists in cells as a double-stranded duplex molecule, whereas RNA, which is composed of very similar nucleotides linked together in the same way, does not form a double-stranded duplex in cells. Why not? If you were to place complementary single-strands of RNA in a test tube, would they spontaneously form duplex molecules? (70) |
|
|
|
3.3.3a The two strands of DNA molecule are held together through hydrogen bonds between nucleotide bases. Which of the following best describes this base pairing in DNA? (70) |
3.3.3a The two strands of DNA molecule are held together through hydrogen bonds between nucleotide bases. Which of the following best describes this base pairing in DNA? (70) |
|
|
|
3.3.3b If the sequence of one chain of DNA double helix is TAACGTA, the sequence of its partner strand in the double helix must be: (70) |
3.3.3b If the sequence of one chain of DNA double helix is TAACGTA, the sequence of its partner strand in the double helix must be: (70) |
|
|
|
3.4.1a Carbohydrates are used for: (70) |
3.4.1a Carbohydrates are used for: (70) |
|
|
|
3.4.1b A carbohydrate is any molecule that contains carbon, hydrogen, and oxygen in the ratio _____. (70) |
3.4.1b A carbohydrate is any molecule that contains carbon, hydrogen, and oxygen in the ratio _____. (70) |
|
|
|
3.4.2a Which of the following carbohydrates is NOT found in plants? (70) |
3.4.2a Which of the following carbohydrates is NOT found in plants? (70) |
|
|
|
3.4.2b The amylase enzyme present in the cells of your body can break the bonds between the glucose monomers in starch, but it cannot break the bonds between the glucose monomers in cellulose. Explain why the same enzyme that breaks down starch cannot break down cellulose. (70) |
The amylase enzyme present in the cells of your body can break the bonds between the glucose monomers in starch, but it cannot break the bonds between the glucose monomers in cellulose. Explain why the same enzyme that breaks down starch cannot break down cellulose. (70) |
|
|
|
3.5.1a A characteristic common to fat molecules is that they: (70) |
3.5.1a A characteristic common to fat molecules is that they: (70) |
|
|
|
3.5.1b A saturated animal fat differs from an unsaturated fat in that it: (70) |
3.5.1b A saturated animal fat differs from an unsaturated fat in that it: (70) |
|
|
|
3.5.2a Lipids are used for: (70) |
3.5.2a Lipids are used for: (70) |
|
|
|
3.5.2b Unlike a phospholipid, cholesterol has: (70) |
3.5.2b Unlike a phospholipid, cholesterol has: (70) |
|

