![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
172 Cards in this Set
- Front
- Back
|
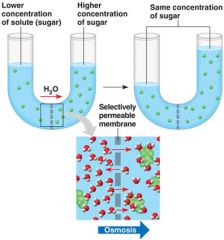
Osmosis
|
is the diffusion of water across a differentially (selectively) permeable membrane. Osmotic pressure is the pressure that develops in a such a system due to osmosis.
|
|
|
Tonicity
|
is the strength of a solution with respect to osmotic pressure. Isotonic solutions occur where the relative solute concentrations of two solutions are equal. hypotonic solution has a solute concentration that is less than another solution. hypertonic solution has a solute concentration that is higher than another solution.
|
|
|
Carrier proteins
|
are membrane proteins that combine with and transport only one type of molecule or ion. they are believed to undergo a change in shape to move the molecule across the membrane.
|
|
|
facilitated transport
|
is the transport of a specific solute down or with its concentration gradient, facilitated by a carrier protein;
|
|
|
Active transport
|
is transport of a specific solute across plasma membranes up or against from low to high its concentration through use of cellular energy (ATP). Active transport requires both carrier proteins and ATP. therefore, cells must have high number of mitochondria near membranes where active transport occurs. Proteins involved in active transport are often called pump.
|
|
|
Exocytosis
|
a vesicle formed by the golgi apparatus fuses with the plasma membrane as secretion occurs.
|
|
|
Endocytosis
|
cells take in substances by vesicle formation as plasma membrane pinches off by either phagocytosis, pinocytosis, or receptor mediated endocytosis.
|
|
|
phagocytosis
|
cells engulf large particles eg bacteria forming endocytic vesicle.
|
|
|
pinocytosis
|
occurs when vesicles form around a liquid or very small particles
|
|
|
Kinetic energy
|
is energy of motion. Potential energy is stored energy.
|
|
|
Two laws of thermodynamics
|
first law : law of conservation of energy. Energy cannot be created or destroyed but it can be changed from one form to another.
second law: energy cannot be changed from one form into another without a loss of usable energy. Heat is a form of energy that dissipates into the environment; heat can never be converted back to another form of energy. |
|
|
Entrop
|
Every energy transformation makes the universe less organized and more disordered; entropy is the term used to indicate the relative amount of disorganization. Energy conversions result in heat, therefore, the entropy of the universe is always increasing.
|
|
|
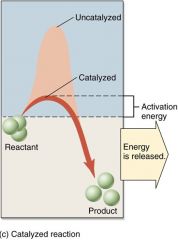
Energy of activation
|
is the energy that must be added to cause molecules to react; without an enzyme this energy can be provided by heat.
|
|
|
Active site
|
a small region on the surface of the enzyme where the substrate binds.
|
|
|
Factors affecting enzymatic speed
|
Substrate concentration.
Temperature and pH: Enzyme activity declines rapidly when enzyme is denatured at a certain temperature due to a change in shape of the enzyme. Enzyme concentration : the amount of active enzyme can regulate the rate of an enzymatic reaction. Coenzyme is an organic cofactor which assists the enzyme |
|
|
Enzyme Inhibition
|
occurs when a substance binds to an enzyme and decreases its activity; normally, enzyme inhibition is reversible.
|
|
|
Competitive inhibition
|
the substrate and the inhibitor are both able to bind to the enzyme's active site
|
|
|
in noncompetitive inhibition
|
the inhibitor binds to the enzyme at a location other than the active site ( the allosteric site) changing the shape of the ezyme and rendering it unable to bind to its substrate. Competitive and noncompetitive inhibitions ae both examples of feedback inhibition.
|
|
|
Oxidation / reduction
|
In oxidation-reduction (redox) reactions, electrons pass from one molecule to another.
Oxidation is the loss of electrons. Reduction is the gain of electrons. Both reactions occur at the same time because one molecule accepts electrons given up by another molecule. |
|
|
Photosynthesis
|
Photosynthesis uses energy to combine carbon dioxide and water to produce glucose in the formula:
6 CO2 + 6 H2O + energy = C6H12O6 + 6 O2
Photosynthesis uses energy to combine carbon dioxide and water to produce glucose in the formula: 6 CO2 + 6 H2O + energy = C6H12O6 + 6 O2 |
|
|
Cellular Respiration
|
The overall equation for cellular respiration is opposite that of photosynthesis:
C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy
When NAD removes hydrogen atoms (H+ + e-) during cellular respiration, the substrate has lost electrons and is therefore oxidized. At the end of cellular respiration, glucose has been oxidized to carbon dioxide and water and ATP molecules have been produced. In metabolic pathways, most oxidations involve the coenzyme NAD+ (nicotinamide adenine dinucleotide); the molecule accepts two electrons but only one hydrogen ion: NAD+ + 2e- + H+ = NADH |
|
|
Electron Transport Chain
|
Both photosynthesis and respiration use an electron transport chain consisting of membrane-bound carriers that pass electrons from one carrier to another.
High-energy electrons are delivered to the system and low-energy electrons leave it. The overall effect is a series of redox reactions; every time electrons transfer to a new carrier, energy is released for the production of ATP |
|
|
ATP Production
|
ATP synthesis is coupled to the electron transport system.
In both mitochondria and chloroplasts, carriers of electron transport systems are located within a membrane. H+ ions (protons) collect on one side of the membrane because they are pumped there by specific proteins. The electrochemical gradient thus established across the membrane is used to provide energy for ATP production. Enzymes and their carrier proteins, called ATP synthase complexes, span the membrane; each complex contains a channel that allows H+ ions to flow down their electrochemical gradient. In photosynthesis, energized electrons lead to the pumping of hydrogen ions across the thylakoid membrane; as hydrogen ions flow through the ATP synthase complex, ATP is formed. During cellular respiration, glucose breakdown provides energy for a hydrogen ion gradient on the inner membrane of the mitochondria that also couples hydrogen ion flow with ATP formation. |
|
|
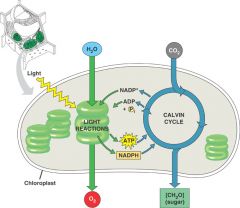
Photosynthesis
|
Carbon dioxide enters a leaf through small openings called stomata.
Carbon dioxide and water diffuse into the chloroplasts, the organelles that carry on photosynthesis. In chloroplasts, a double membrane encloses a fluid-filled space called the stroma. An internal membrane system within the stroma forms flattened sacs called thylakoids, which in some cases are organized into stacks to form grana. Spaces within all thylakoids are connected to form an inner compartment, the thylakoid space. Chlorophyll and other pigments involved in absorption of solar energy reside within thylakoid membranes; these pigments absorb solar energy, and energize electrons prior to reduction of CO2 to a carbohydrate. |
|
|
Light reactions
|
Light reactions are the energy-capturing reactions.
Chlorophyl within thylakoid membranes absorbs solar energy and energizes electrons. When energized electrons move down an electron transport chain, energy is captured and used for ATP production. Energized electrons are also taken up by NADP+, converting it to NADPH. |
|
|
Calvin cycle reactions
|
These reactions take place in the stroma; the reactions can occur in either the presence or the absence of light.
These are synthetic reactions that use NADPH and ATP to reduce CO2. |
|
|
Light reaction
|
Two electron pathways operate in the thylakoid membrane: the noncyclic pathway and the cyclic pathway.
Both pathways produce ATP; only the noncyclic pathway also produces NADPH. ATP production during photosynthesis is called photophosphorylation; therefore these pathways are also known as cyclic and noncyclic photophosphorylation. |
|
|
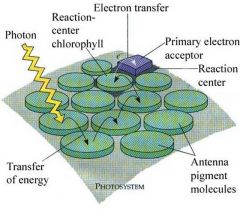
Photosystem 2
|
This pathway occurs in the thylakoid membranes and requires participation of two light-gathering units: photosystem I (PS I) and photosystem II (PS II).
A photosystem is a photosynthetic unit comprised of a pigment complex and an electron acceptor; solar energy is absorbed and high-energy electrons are generated. Each photosystem has a pigment complex of chlorophyll a, chlorophyll b, carotenoid, and electron acceptor molecules. Absorbed energy is passed from one pigment molecule to another until concentrated in reaction-centerchlorophyll a molecules. Electrons in reaction-center chlorophyll a become excited, and escape to the electron-acceptor molecule. The noncyclic pathway begins with PSII; electrons move from H2O through PS II to PS I and then on to NADP+. The PS II pigment complex absorbs solar energy; high-energy electrons (e-) leave the reaction-center chlorophyll a molecule. PS II takes replacement electrons from H2O, which splits, releasing O2 and H+ ions: H2O = 2 H+ + 2 e- + ½ O2. Oxygen is released as oxygen gas (O2). The H+ ions temporarily stay within the thylakoid space and contribute to a H+ ion gradient. As H+ flow down electrochemical gradient through ATP synthase complexes, chemiosmosis occurs. Low-energy electrons leaving the electron transport system enter PS I. When the PS I pigment complex absorbs solar energy, high-energy electrons leave reaction-center chlorophyll a and are captured by an electron acceptor. The electron acceptor passes them on to NADP+. NADP+ takes on an H+ to become NADPH: NADP+ + 2 e- + H+ = NADPH. NADPH and ATP (produced by noncyclic-flow electrons in the thylakoid membrane) are used by enzymes in the stroma during the light-independent (dark) reactions. |
|
|
Photosystem 1
|
The cyclic electron pathway begins when the PS I antenna complex absorbs solar energy.
High-energy electrons leave PS I reaction-center chlorophyll a molecule. Before they return, the electrons enter and travel down an electron transport chain. Electrons pass from a higher to a lower energy level. Energy released is stored in the form of a hydrogen (H+) gradient. When hydrogen ions flow down their electrochemical gradient through ATP synthase complexes, ATP production occurs. The electrons return to PSI rather than move on to NADP+--this is why it is called cyclic and also why no NADPH is produced. It is possible that in plants, the cyclic flow of electrons is utilized only when CO2 is in such limited supply that carbohydrate is not being produced. |
|
|
Thylakoid membrane
|
PS II consists of a pigment complex and electron-acceptor molecules; it oxidizes H2O and produces O2.
The electron transport system consists of cytochrome complexes and transports electrons and pumps H+ ions into the thylakoid space. PS I has a pigment complex and electron-acceptor molecules; it is associated with an enzyme that reduces NADP+ to NADPH. ATP synthase complex has an H+ channel and ATP synthase; it produces ATP. |
|
|
ATP production
|
The thylakoid space acts as a reservoir for H+ ions; each time H2O is split, two H+ remain.
Electrons move carrier-to-carrier, giving up energy used to pump H+ from the stroma into the thylakoid space. Flow of H+ from high to low concentration across thylakoid membrane provides energy to produce ATP from ADP + P by using an ATP synthase enzyme. This is called chemiosmosis because ATP production is tied to an electrochemical (H+) gradient. |
|
|
Calvin cycle reactions
|
The Calvin cycle is a series of reactions producing carbohydrates; these reactions follow the light reactions.CO2 fixation is the attachment of CO2 to an organic compound called RuBP.
RuBP (ribulose bisphosphate) is a five-carbon molecule that combines with carbon dioxide; the resulting 6-carbon molecule then splits into two 3-carbon molecules. The enzyme RuBP carboxylase (rubisco) speeds this reaction; this enzyme comprises 20–50% of the protein content of chloroplasts--it is an unusually slow enzyme. With the reduction of carbon dioxide, a 3PG (3-phosphoglycerate) molecule forms. Each of two 3PG molecules undergoes reduction to G3P (glyceraldehyde-3-phosphate) in two steps. Light-dependent reactions provide NADPH (electrons) and ATP (energy) to reduce 3PG to G3P. For every three turns of the Calvin cycle, five molecules of G3P are used to re-form three molecules of RuBP. This reaction also uses ATP produced by the light reactions. |
|
|
Cellular respiration
|
Cellular respiration involves various metabolic pathways that break down carbohydrates and other metabolites with the concomitant buildup of ATP.
Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. Cellular respiration usually involves the complete breakdown of glucose into CO2 and H2O. The net equation for glucose breakdown is: C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. Electrons are removed from substrates and received by oxygen, which combines with H+ to become water. Glucose is oxidized and O2 is reduced. The buildup of ATP is an endergonic reaction (i.e., requires energy). The reactions of cellular respiration allow energy in glucose to be released slowly; therefore ATP is produced gradually. In contrast, if glucose were broken down rapidly, most of its energy would be lost as non-usable heat. The breakdown of glucose yields synthesis of 36 or 38 ATP (depending on certain conditions); this preserves about 39% of the energy available in glucose. |
|
|
NAD+ and FAD
|
Each metabolic reaction in cellular respiration is catalyzed by a specific enzyme.
As a metabolite is oxidized, NAD+ (nicotinamide adenine dinucleotide) accepts two electrons and a hydrogen ion (H+); this results in NADH + H+. Electrons received by NAD+ and FAD are high-energy electrons and are usually carried to the electron transport chain. NAD+ is a coenzyme of oxidation-reduction since it both accepts and gives up electrons; thus, NAD+ is sometimes called a redox coenzyme Only a small amount of NAD+ is needed in cells because each NAD+ molecule is used repeatedly. FAD coenzyme of oxidation-reduction can replace NAD+; FAD accepts two electrons and two hydrogen ions to become FADH2. |
|
|
Phase one of cellular respiration
|
Glycolysis is the breakdown of glucose in the cytoplasm into two molecules of pyruvate.
Enough energy is released for an immediate yield of two ATP. Glycolysis takes place outside the mitochondria and does not utilize oxygen; it is therefore an anaerobic process. |
|
|
Phase two of cellular respiration
|
In the preparatory (prep) reaction, pyruvate enters a mitochondrion and is oxidized to a two-carbon acetyl group and CO2 is removed; this reaction occurs twice per glucose molecule
|
|
|
Phase tree of cellular respiration
|
citric acid cycle or kreb's cycle
occurs in the matrix of the mitochondrion and produces NADH and FADH2; is a series of reactions that gives off CO2 and produces one ATP; turns twice because two acetyl-CoA molecules enter the cycle per glucose molecule; produces two immediate ATP molecules per glucose molecule. |
|
|
Phase four of cellular respiration
|
The electron transport chain:
is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed from carrier to carrier until received by oxygen; passes electrons from higher to lower energy states, allowing energy to be released and stored for ATP production; accounts for 32 or 34 ATP, depending on certain cell conditions. |
|
|
Inside the mitochondria
|
The next reactions of cellular respiration involve the preparatoryreaction, the citric acid cycle, and the electron transport chain.
In these reactions, the pyruvate from glycolysis is broken down completely to CO2 and H2O. CO2 and ATP are transported out of the mitochondria into the cytoplasm. The H2O can remain in the mitochondria or within the cell, or it can enter the blood and be excreted by the kidneys. A mitochondrion has a double membrane with an intermembrane space (between the outer and inner membrane). Cristae are the inner folds of membrane that jut into the matrix, the innermost compartment of a mitochondrion that is filled with a gel-like fluid. The prep reaction and citric acid cycle enzymes are in the matrix; the electron transport chain is in the cristae. Most of the ATP produced in cellular respiration is produced in the mitochondria; therefore, mitochondria are often called the "powerhouses" of the cell. |
|
|
Preparatory Reaction
|
The preparatory reaction connects glycolysis to the citric acid cycle.
In this reaction, pyruvate is converted to a two-carbon acetyl group, and is attached to coenzyme A, resulting in the compound acetyl-CoA. This redox reaction removes electrons from pyruvate by a dehydrogenase enzyme, using NAD+ as a coenzyme. This reaction occurs twice for each glucose molecule. |
|
|
Kreb's cycle
|
The citric acid cycle occurs in the matrix of mitochondria.
The cycle begins by the addition of a two-carbon acetyl group to a four-carbon molecule, forming a six-carbon citrate (citric acid) molecule. In the subsequent reactions, at three different times two electrons and one hydrogen ion are accepted by NAD+, forming NADH. At one time, two electrons and one hydrogen ion are accepted by FAD, forming FADH2. NADH and FADH2 carry these electrons to the electron transport chain. Some energy is released and is used to synthesize ATP by substrate-level phosphorylation. One high-energy metabolite accepts a phosphate group and transfers it to convert ADP to ATP. The citric acid cycle turns twice for each original glucose molecule. The products of the citric acid cycle (per glucose molecule) are 4 CO2, 2 ATP, 6 NADH and 2 FADH2. The six carbon atoms in the glucose molecule have now become the carbon atoms of six CO2 molecules, two from the prep reaction and four from the citric acid cycle. |
|
|
Electron transport chain
|
The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another.
Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in the center. NADH and FADH2 carry the electrons to the electron transport system.. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. At each sequential redox reaction, energy is released to form ATP molecules. Because O2 must be present for the proteins to work, this process is also called oxidative phosphorylation. Oxygen serves as the terminal electron acceptor and combines with hydrogen ions to form water. By the time electrons are received by O2, three ATP have been made. When FADH2 delivers electrons to the electron transport system, two ATP are formed by the time the electrons are received by O2. Coenzymes and ATP undergo recycling. Cell needs a limited supply of coenzymes NAD+ and FAD because they constantly recycle. Once NADH delivers electrons to the electron transport chain, it can accept more hydrogen atoms. ADP and phosphate also recycle. Efficiency of recycling NAD+, FAD, and ADP eliminates the need to continuously synthesize them anew. |
|
|
Cristae of Mitochondrion
|
The electron transport chain consists of three protein complexes and two protein mobile carriers that transport electrons.
The three protein complexes include NADH-Q reductase complex, the cytochrome reductasecomplex, and the cytochrome oxidase complex; the two protein mobile carriers are coenzyme Q and cytochrome c. Energy released from the flow of electrons down the electron transport chain is used to pump H+ ions, which are carried by NADH and FADH2, into intermembrane space. Accumulation of H+ ions in this intermembrane space creates a strong electrochemical gradient. ATP synthase complexes are channel proteins that serve as enzymes for ATP synthesis. As H+ ions flow from high to low concentration, ATP synthase synthesizes ATP by the reaction: ADP + P = ATP. Chemiosmosis is the term used for ATP production tied to an electrochemical (H+) gradient across a membrane. Respiratory poisons confirm the chemiosmotic nature of ATP synthesis (i.e., a poison that inhibits ATP synthesis increases the H+ gradient). Once formed, ATP molecules diffuse out of the mitochondrial matrix through channel proteins. ATP is the energy currency for all living things; all organisms must continuously produce high levels of ATP to survive. |
|
|
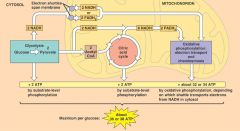
Energy Yield From Glucose Metabolism
|
Substrate-Level Phosphorylation
Per glucose molecule, there is a net gain of two ATP from glycolysis in cytoplasm. The citric acid cycle in the matrix of the mitochondria produces two ATP per glucose. Thus, a total of four ATP are formed by substrate-level phosphorylation outside of the electron transport chain. Electron Transport Chain and Chemiosmosis Most of the ATP is produced by the electron transport chain and chemiosmosis. Per glucose, ten NADH and two FADH2 molecules provide electrons and H+ ions to the electron transport chain. For each NADH formed within the mitochondrion, three ATP are produced. For each FADH2 formed by the citric acid cycle, two ATP are produced. For each NADH formed outside mitochondria by glycolysis, two ATP are produced as electrons are shuttled across the mitochondrial membrane by an organic molecule and delivered to FAD. |
|
|
Kreb's cycle
|
The citric acid cycle occurs in the matrix of mitochondria.
The cycle begins by the addition of a two-carbon acetyl group to a four-carbon molecule, forming a six-carbon citrate (citric acid) molecule. In the subsequent reactions, at three different times two electrons and one hydrogen ion are accepted by NAD+, forming NADH. At one time, two electrons and one hydrogen ion are accepted by FAD, forming FADH2. NADH and FADH2 carry these electrons to the electron transport chain. Some energy is released and is used to synthesize ATP by substrate-level phosphorylation. One high-energy metabolite accepts a phosphate group and transfers it to convert ADP to ATP. The citric acid cycle turns twice for each original glucose molecule. The products of the citric acid cycle (per glucose molecule) are 4 CO2, 2 ATP, 6 NADH and 2 FADH2. The six carbon atoms in the glucose molecule have now become the carbon atoms of six CO2 molecules, two from the prep reaction and four from the citric acid cycle. |
|
|
Electron transport chain
|
The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another.
Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in the center. NADH and FADH2 carry the electrons to the electron transport system.. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. At each sequential redox reaction, energy is released to form ATP molecules. Because O2 must be present for the proteins to work, this process is also called oxidative phosphorylation. Oxygen serves as the terminal electron acceptor and combines with hydrogen ions to form water. By the time electrons are received by O2, three ATP have been made. When FADH2 delivers electrons to the electron transport system, two ATP are formed by the time the electrons are received by O2. Coenzymes and ATP undergo recycling. Cell needs a limited supply of coenzymes NAD+ and FAD because they constantly recycle. Once NADH delivers electrons to the electron transport chain, it can accept more hydrogen atoms. ADP and phosphate also recycle. Efficiency of recycling NAD+, FAD, and ADP eliminates the need to continuously synthesize them anew. |
|
|
Cristae of Mitochondrion
|
The electron transport chain consists of three protein complexes and two protein mobile carriers that transport electrons.
The three protein complexes include NADH-Q reductase complex, the cytochrome reductasecomplex, and the cytochrome oxidase complex; the two protein mobile carriers are coenzyme Q and cytochrome c. Energy released from the flow of electrons down the electron transport chain is used to pump H+ ions, which are carried by NADH and FADH2, into intermembrane space. Accumulation of H+ ions in this intermembrane space creates a strong electrochemical gradient. ATP synthase complexes are channel proteins that serve as enzymes for ATP synthesis. As H+ ions flow from high to low concentration, ATP synthase synthesizes ATP by the reaction: ADP + P = ATP. Chemiosmosis is the term used for ATP production tied to an electrochemical (H+) gradient across a membrane. Respiratory poisons confirm the chemiosmotic nature of ATP synthesis (i.e., a poison that inhibits ATP synthesis increases the H+ gradient). Once formed, ATP molecules diffuse out of the mitochondrial matrix through channel proteins. ATP is the energy currency for all living things; all organisms must continuously produce high levels of ATP to survive. |
|
|
Energy Yield From Glucose Metabolism
|
Substrate-Level Phosphorylation
Per glucose molecule, there is a net gain of two ATP from glycolysis in cytoplasm. The citric acid cycle in the matrix of the mitochondria produces two ATP per glucose. Thus, a total of four ATP are formed by substrate-level phosphorylation outside of the electron transport chain. Electron Transport Chain and Chemiosmosis Most of the ATP is produced by the electron transport chain and chemiosmosis. Per glucose, ten NADH and two FADH2 molecules provide electrons and H+ ions to the electron transport chain. For each NADH formed within the mitochondrion, three ATP are produced. For each FADH2 formed by the citric acid cycle, two ATP are produced. For each NADH formed outside mitochondria by glycolysis, two ATP are produced as electrons are shuttled across the mitochondrial membrane by an organic molecule and delivered to FAD. |
|
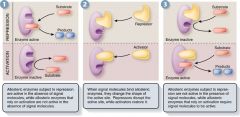
Active site of enzyme
|
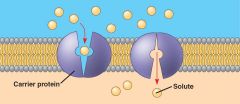
Facilitated Diffusion
|
|
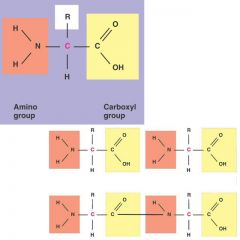
Amino Acids
|
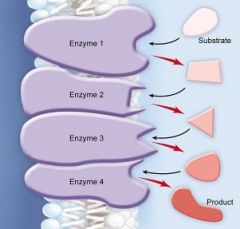
Biochemical pathway
|
|
Osmosis
|
Catalyzed reaction
|
|
Cell membrane
|
Cell respiration
|
|
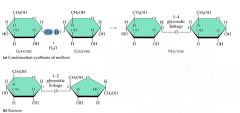
dehydration synthesis
|
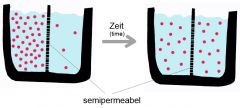
Diffusion
|
|
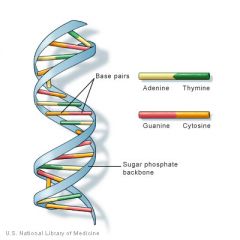
DNA
|
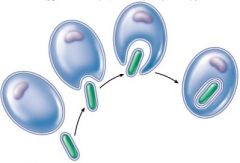
Endosymbiosis
|
|
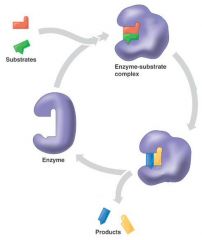
Enzyme in action
|
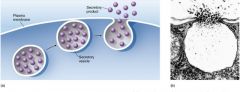
Exocytosis
|
|
|
Electron Transport Chain
|
Glyclosis
|
|
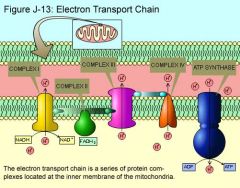
Electron Transport Chain
|
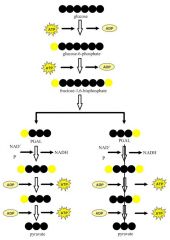
Glyclosis
|
|
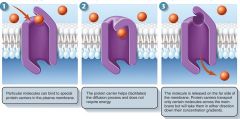
Facilitated diffusion
|
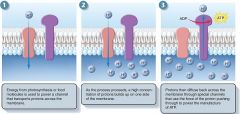
Proton Pump
|
|
Function of cell
|
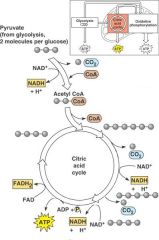
Krebs Cycle
|
|

Lipid Bilayer
|
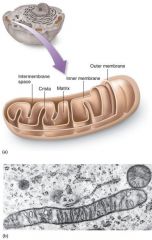
mitochondria
|
|
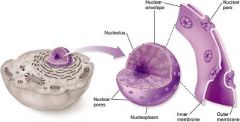
Nucleus
|
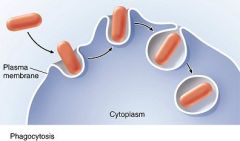
Phagocytosis
|
|
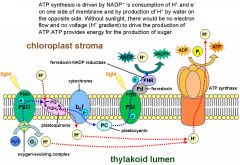
Photosynthesis
|
Overview of photosynthesis
|
|
Photosystems
|

Plasma membrane function
|
|
Plasma Membrane Functions
|
secondary structure
|
|
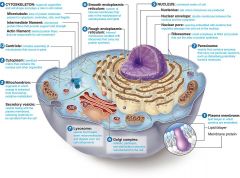
Animal Cell ( Eukaryotic )
|
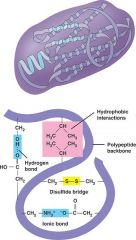
Tertiary structure
|
|
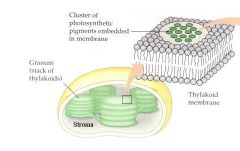
Thylakoid Membrane
|
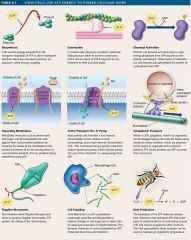
Uses of atp
|
|
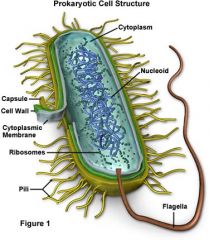
Prokaryotic cell
|
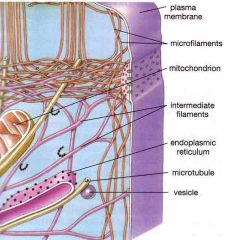
Cytoskeleton
|
|
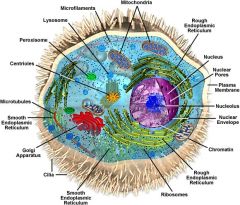
Eukaryotic cell
|
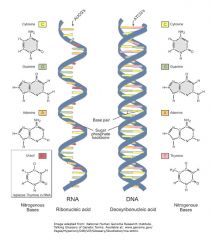
Difference between RNA AND DNA
|
|
hydrogen bonds
|
prokaryote cell
|
|
cytoskeleton
|
dehydration / hydrolysis
|
|
peptide bond
|
Eukaryotic
|
|
macromolecule
|
Ionic bonds
|
|

Properties of water
|
Protein Structures
|
|
(un) saturated fats
|
polarity / water molecules
|
|
|
atom
|
Elements consist of tiny particles called atoms. An atom is the smallest unit of an element that displays the properties of the element.One or two letters (e.g., H, Na) create the atomic symbol of the element.
|
|
|
atomic number
|
The atomic mass of an atom depends on the presence of certain subatomic particles.
Atoms contain specific numbers of protons, neutrons, and electrons. Protons and neutrons are in the nucleus of an atom; electrons move around the nucleus. Protons are positively charged particles; neutrons have no charge; both have 1 atomic mass unit (amu) of weight. Electrons are negatively charged particles located in orbitals outside the nucleus. All atoms of an element have the same number of protons, called the atomic number of the element. |
|
|
Isotopes
|
Isotopes are atoms of the same element that differ in the number of neutrons (and therefore have different atomic masses). For example, carbon-12 has 6 protons and 6 neutrons, carbon-14 has 6 protons and 8 neutrons.
A carbon atom with 8 rather than 6 neutrons is unstable; it releases energy and subatomic particles and is thus a radioactive isotope. Because the chemical behavior of a radioactive isotope is the same as a stable isotope of a particular element, low levels of the radioactive isotope (e.g., radioactive iodine or glucose) allow researchers to trace the location and activity of the element in living tissues; these isotopes are called tracers. |
|
|
electron
|
Electrons occupy orbitals within various energy levels (or electron shells) near or distant from the nucleus of the atom. The farther the orbital from the nucleus, the higher the energy level.
An orbital is a volume of space where an electron is most likely to be found; an orbital can contain no more than 2 electrons. When atoms absorb energy during photosynthesis, electrons are boosted to higher energy levels. When the electrons return to their original energy level, the released energy is converted into chemical energy. |
|
|
octet rule
|
The innermost shell of an atom is complete with 2 electrons; all other shells are complete with 8 electrons. This is called the octet rule.
Atoms will give up, accept, or share electrons in order to have 8 electrons in a electron shell. |
|
|
molecule
|
A molecule is the smallest part of a compound that has the properties of the compound.
Electrons possess energy, and bonds that exist between atoms in molecules therefore contain energy |
|
|
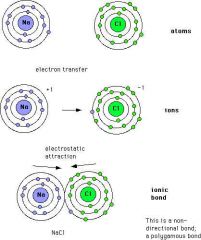
ionic bonding
|
An ionic bond forms when electrons are transferred from one atom to another atom.
By losing or gaining electrons, atoms fill outer shells, and are more stable (the octet rule). Example: sodium loses an electron and therefore has a positive charge; chlorine gains an electron to give it a negative charge. Such charged particles are called ions. Attraction of oppositely charged ions holds the two atoms together in an ionic bond. A salt (e.g., NaCl) is an example of an ionically-bonded compound. |
|
|
covalent bonding
|
Covalent bonds result when two atoms share electrons so each atom has an octet of electrons in the outer shell (or, in the case of hydrogen, 2 electrons).
Hydrogen can give up an electron to become a hydrogen ion (H+) or share an electron with another atom to complete its shell with 2 electrons. The structural formula of a compound indicates a shared pair of electrons by a line between the two atoms; e.g., single covalent bond (H–H), double covalent bond (O=O), and triple covalent bond (N = N). Each line between the atoms represents a pair of electrons. |
|
|
nonpolar covalent bonds
|
nonpolar covalent bonds, sharing of electrons is equal, i.e., the electrons are not attracted to either atom to a greater degree.
|
|
|
Polar Covalent Bonds
|
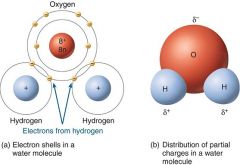
With polar covalent bonds, the sharing of electrons is unequal.
In a water molecule (H2O), sharing of electrons by oxygen and hydrogen is not equal; the oxygen atom with more protons attracts the electrons closer to it, and thus dominates the H2O association. Attraction of an atom for electrons in a covalent bond is called the electronegativity of the atom; an oxygen atom is more electronegative than a hydrogen atom. Oxygen in a water molecule, more attracted to the electron pair, assumes a partial negative charge. |
|
|
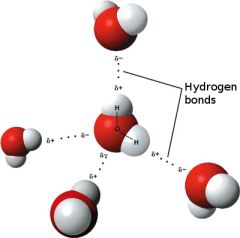
Hydrogen Bonding
|
A hydrogen bond is a weak attractive force between the slightly positive charge of the hydrogen atom of one molecule and slightly negative charge of another atom (e.g., oxygen, nitrogen) in another or the same molecule.
Many hydrogen bonds taken together are relatively strong. Hydrogen bonds between and within complex biological molecules (e.g., DNA, proteins) help maintain their proper structure and function. |
|
|
Properties of water
|
Water has a high heat capacity
Water has a high heat of vaporization. Water is a solvent. Water molecules are cohesive and adhesive. Water has a high surface tension. Unlike most substances, frozen water is less dense than liquid water. |
|
|
Hydrophobic
|
Nonionized and nonpolar molecules that cannot attract water are hydrophobic ("water fearing").
|
|
|
hydrophilic
|
Ionized or polar molecules attracted to water are hydrophilic ("water loving").
|
|
|
Cohesion
|
Cohesion allows water to flow freely without molecules separating.
|
|
|
Adhesion
|
Adhesion is ability to adhere to polar surfaces; water molecules have positive and negative poles.
|
|
|
Acids
|
Acid molecules dissociate in water, releasing hydrogen (H+) ions: HCl → H+ + Cl-.
|
|
|
Bases
|
Bases are molecules that take up hydrogen ions or release hydroxide ions. NaOH → Na+ + OH-.
|
|
|
pH scale
|
The pH scale indicates acidity and basicity (alkalinity) of a solution.
pH is the measurement of free hydrogen ions, expressed as a negative logarithm of the H+ concentration (-log [H+]). pH values range from 0 (100 moles/liter; most acidic) to 14 (1014 moles/liter; most basic). |
|
|
Buffers
|
Buffers keep pH steady and within normal limits in living organisms..
|
|
|
Organic Molecules
|
Organic molecules contain carbon and hydrogen atoms bonded to other atoms.
Four types of organic molecules (biomolecules) exist in organisms: carbohydrates, lipids, proteins, and nucleic acids. |
|
|
Hydrocarbons
|
Hydrocarbons are chains of carbon atoms bonded exclusively to hydrogen atoms;Carbon atoms can form double or triple bonds with certain atoms (carbon, nitrogen).
|
|
|
Functional Group
|
Functional groups are clusters of specific atoms bonded to the carbon skeleton with characteristic structure and functions.
|
|
|
Isomers
|
Isomers are molecules with identical molecular formulas but different arrangements of their atoms (e.g., glyceraldehyde and dihydroxyacetone).
|
|
|
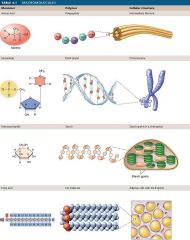
Macromolecules
|
Carbohydrates, lipids, proteins, and nucleic acids are called macromolecules because of their large size.
The largest macromolecules are called polymers, constructed by linking many of the same type of small subunits, called monomers. Examples: amino acids (monomers) are linked to form a protein (polymer); many nucleotides (monomers) are linked to form a nucleic acid (polymer). |
|
|
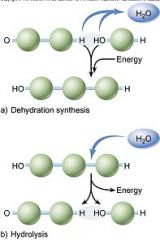
Dehydration reactions
|
Cellular enzymes carry out dehydration reactions to synthesize macromolecules. In a dehydration reaction, a water molecule is removed and a covalent bond is made between two atoms of the monomers.
In a dehydration reaction, a hydroxyl (—OH) group is removed from one monomer and a hydrogen (—H) is removed from the other. This produces water, and, because the water is leaving the monomers, it is a dehydration reaction. |
|
|
Hydrolysis
|
Hydrolysis ("water breaking") reactions break down polymers in reverse of dehydration; a hydroxyl (—OH) group from water attaches to one monomer and hydrogen (—H) attaches to the other.
|
|
|
Enzymes
|
Enzymes are molecules that speed up chemical reactions by bringing reactants together; an enzyme may even participate in the reaction but is not changed by the reaction
|
|
|
Monosaccharides
|
Monosaccharides are simple sugars with a backbone of 3 to 7 carbon atoms.
Disaccharides contain two monosaccharides joined by a dehydration reaction. |
|
|
Polysaccharides
|
Polysaccharides are polymers of monosaccharides. They are not soluble in water and do not pass through the plasma membrane of the cell.
Starch, found in many plants, is a straight chain of glucose molecules with relatively few side branches. Amylose and amylopectin are the two forms of starch found in plants. Glycogen is a highly branched polymer of glucose with many side branches. It is the storage form of glucose in animals. Cellulose is a polymer of glucose which forms microfibrils, the primary constituent of plant cell walls. Chitin is a polymer of glucose with an amino group attached to each glucose. |
|
|
Lipids
|
Lipids are hydrocarbons that are insoluble in water because they lack polar groups.
Fat provides insulation and energy storage in animals. Phospholipids form plasma membranes and steroids are important cell messengers. Waxes have protective functions in many organisms. |
|
|
Fatty Acids
|
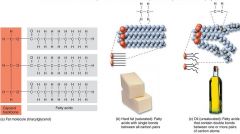
A fatty acid is a long hydrocarbon chain with a carboxyl (acid) group at one end.
Saturated fatty acids have no double bonds between their carbon atoms. Unsaturated fatty acids have double bonds in the carbon chain where there are less than two hydrogens per carbon atom. Fats contain saturated fatty acids and are solid at room temperature (e.g., butter). Oils contain unsaturated fatty acids and are liquid at room temperature. |
|
|
Phospholipids
|
Phospholipids are constructed like neutral fats except that the third fatty acid is replaced by a polar (hydrophilic) phosphate group; the phosphate group usually bonds to another organic group (designated by R).
The hydrocarbon chains of the fatty acids become the nonpolar (hydrophobic) tails. Phospholipids arrange themselves in a double layer in water, so the polar heads face toward water molecules and nonpolar tails face toward one other, away from water molecules. This property enables phospholipids to form an interface or separation between two solutions (e.g., the interior and exterior of a cell); the plasma membrane is a phospholipid bilayer. |
|
|
Protein Functions
|
Support proteins include keratin, which makes up hair and nails, and collagen fibers, which support many of the body's structures (e.g., ligaments, tendons, skin).
Enzymes are proteins that act as organic catalysts to accelerate chemical reactions within cells. Transport functions include channel and carrier proteins in the plasma membrane, and hemoglobin that transports oxygen in red blood cells. Defense functions include antibodies that prevent infection. Hormones are regulatory proteins that influence the metabolism of cells. For example, insulin regulates glucose content of blood and within cells. Motion within cells and by muscle contraction is provided by the proteins myosin and actin. |
|
|
Amino Acids: Building Blocks of Proteins
|
Amino acids contain an acidic group (— COOH) and an amino group (—NH2).
Amino acids differ according to their particular R group, ranging from single hydrogen to complicated ring compounds. There are 20 different amino acids commonly found in cells. |
|
|
Peptides
|
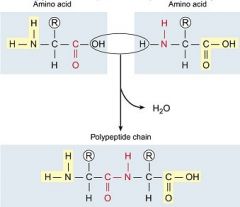
A peptide bond is a covalent bond between two amino acids.
Atoms of a peptide bond share electrons unevenly (oxygen is more electronegative than nitrogen). The polarity of the peptide bond permits hydrogen bonding between different amino acids in a polypeptide. A peptide is two or more amino acids bonded together. Polypeptides are chains of many amino acids joined by peptide bonds. |
|
|
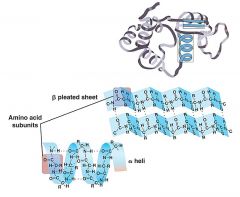
Shape of Protein
|
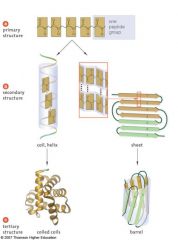
Protein shape determines the function of the protein in the organism; proteins can have up to four levels of structure
The primary structure is the protein's own particular sequence of amino acids. The secondary structure results when a polypeptide coils or folds in a particular way. In a peptide bond, oxygen is partially negative, hydrogen is partially positive. This allows for hydrogen bonding between the C=O of one amino acid and the N—H of another. Hydrogen bonding between every fourth amino acid holds the spiral shape of an a helix. Pleated b sheet polypeptides turn back upon themselves. Hydrogen bonding occurs between extended lengths. Tertiary structure results when proteins are folded, giving rise to the final three-dimensional shape of the protein. This is due to interactions among the R groups of the constituent amino acids. Quaternary structure results when two or more polypeptides combine |
|
|
nucleic acids
|
Nucleic acids are polymers of nucleotides with very specific functions in cells.
DNA (deoxyribonucleic acid) stores the genetic code for its own replication and for the amino acid sequences in proteins. RNA (ribonucleic acid) allows for translation of the genetic code of DNA into the amino acid sequence of proteins; other functions for RNA in the cell exist.Coenzymes are molecules which facilitate enzymatic reactions. ATP (adenosine triphosphate) is a nucleotide used to supply energy for synthetic reactions and other energy-requiring metabolic activities in the cell. |
|
|
Dna and Rna differ how?
|
DNA and RNA differ in the following ways:
Nucleotides of DNA contain deoxyribose sugar; nucleotides of RNA contain ribose. In RNA, the base uracil occurs instead of the base thymine. Both RNA and DNA contain adenine, guanine, and cytosine. DNA is double-stranded with complementary base pairing; RNA is single-stranded. Complementary base pairing occurs where two strands of DNA are held together by hydrogen bonds between purine and pyrimidine bases. The number of purine bases always equals the number of pyrimidine bases. In DNA, thymine is always paired with adenine; cytosine is always paired with guanine. Thus, in DNA: A + G = C + T. Two strands of DNA twist to form a double helix; RNA does not form helices. |
|
|
Cell Theory
|
The cell theory states that all organisms are composed of cells, that cells are the structural and functional unit of organisms, and that cells come only from preexisting cells.
|
|
|
Cell theory
|
The cell theory states that all organisms are composed of cells, that cells are the structural and functional unit of organisms, and that cells come only from preexisting cells.
|
|
|
Cell Size
|
The surface-area-to-volume ratio requires that cells be small.
|
|
|
Microscope
|
Compound light microscopes use light rays focused by glass lenses.
Transmission electron microscopes (TEM) use electrons passing through specimen and focused by magnets. Scanning electron microscopes (SEM) use electrons scanned across metal-coated specimen; secondary electrons given off by metal are collected by a detector. |
|
|
Magnification / Resolution
|
Magnification is a function of wavelength; the shorter wavelengths of electrons allow greater magnification than the longer wavelengths of light rays.
Resolution is the minimum distance between two objects at which they can still be seen as separate objects. |
|
|
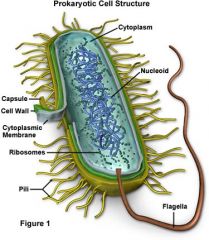
Prokaryotic cells
|
Prokaryotic cells lack a nucleus and are smaller and simpler than eukaryotic cells (which have a nucleus).
|
|
|
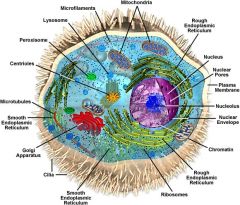
Eukaryotic Cells
|
Eukaryotic cells are members of the domain Eukarya, which includes the protists, fungi, plants, and animals.
A membrane-bounded nucleus houses DNA; the nucleus may have originated as an invagination of the plasma membrane. Eukaryotic cells are much larger than prokaryotic cells, and therefore have less surface area per volume. Eukaryotic cells are compartmentalized; they contain small structures called organelles that perform specific functions. Some eukaryotic cells (e.g., plant cells) have a cell wall containing cellulose; |
|
|
Golgi Apparatus
|
Enzymes within the Golgi apparatus modify the carbohydrates that were placed on proteins in the ER; proteins and lipids are sorted and packaged.
Vesicles formed from the membrane of the outer face of the Golgi apparatus move to different locations in a cell; at the plasma membrane they discharge their contents as secretions, a process called exocytosis because substances exit the cell. |
|
|
Lysosomes
|
Lysosomes are membrane-bounded vesicles produced by the Golgi apparatus.
Lysosomes contain powerful digestive enzymes and are highly acidic. |
|
|
Endoplasmic Reticulum
|
The endoplasmic reticulum (ER) is a system of membrane channels and saccules (flattened vesicles) continuous with the outer membrane of the nuclear envelope.
Rough ER is studded with ribosomes on the cytoplasm side; it is the site where proteins are synthesized and enter the ER interior for processing and modification. Smooth ER is continuous with rough ER but lacks ribosomes; it is a site of various synthetic processes, detoxification, and storage; smooth ER forms transport vesicles. |
|
|
Chromosomes
|
Chromosomes are rodlike structures formed during cell division; composed of coiled or folded chromatin.
|
|
|
Nucleolus
|
The nucleolus is a dark region of chromatin inside the nucleus;
|
|
|
Nuclear envelope / nuclear pores
|
The nucleus is separated from the cytoplasm by the nuclear envelope, which contains nuclear pores to permit passage of substances (e.g., ribosomal subunits, messenger RNA, proteins, etc.) in and out of the nucleus
|
|
|
Ribosomes
|
Ribosomes are the site of protein synthesis in the cell. In eukaryotic cells, ribosomes may occur freely or in groups called polyribosomes.
|
|
|
Peroxisomes
|
Peroxisomes are membrane-bounded vesicles that contain specific enzymes.
|
|
|
Vacuoles
|
Vacuoles are mebranous sacs and are larger than vesicles.
The central vacuole of a plant cell maintins turgor pressure within the cell, stores nutrients and wastes, and degrades organelles as the cell ages. |
|
|
Chloroplast
|
Chloroplasts are membranous organelles (a type of plastid) that serve as the site of photosynthesis.
Photosynthesis is represented by the equation: solar energy + carbon dioxide + water → carbohydrate + oxygen Only plants, algae, and certain bacteria are capable of conducting photosynthesis. The chloroplast is bound by a double membrane organized into flattened disc-like sacs called thylakoids formed from a third membrane; a stack of thylakoids is a granum. |
|
|
Mitochondria
|
Mitochondria are surrounded by a double membrane: the inner membrane surrounds the matrix and is convoluted to form cristae.
Mitochondria contain ribosomes and their own DNA. |
|
|
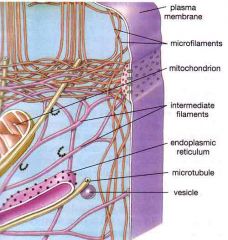
Cytoskeleton
|
The cytoskeleton is a network of connected filaments and tubules; it extends from the nucleus to the plasma membrane in eukaryotes.
|
|
|
Actin Filaments
|
Actin filaments play a structural role, forming a dense complex web just under the plasma membrane
|
|
|
ntermediate Filaments
|
They are rope-like assemblies of fibrous polypeptides.
Some support the nuclear envelope; others support plasma membrane and form cell-to-cell junctions. |
|
|
Microtubules
|
Microtubules are composed of a globular protein tubulin.
Regulation of microtubule assembly is under control of a microtubule organizing center (MTOC): the main MTOC is called a centrosome. |
|
|
Centrioles
|
Centrioles are short cylinders with a ring pattern (9 + 0) of microtubule triplets.
Centrioles serve as basal bodies for cilia and flagella. |
|
|
Cilia
|
Cilia are short, usually numerous hairlike projections that can move in an undulating fashion
|
|
|
Flagella
|
Flagella are longer, usually fewer, projections that move in whip-like fashion
|
|
|
Carbon Fixation
|
Carbon fixation refers to any process through which gaseous carbon dioxide is converted into a solid compound
|
|
|
Protists
|
A eukaryote other than animals, plants and fungi; often single celled.
|
|
|
Peroxisome
|
a membrane-bound compartment in the cell that is responsible for the breakdown of certain types of fatty acids
|
|
|
Tonoplast
|
Membrane surrounding the central vacuole
|
|
|
Plasmodesmata
|
opening in cell walls that function in cell-cell communication.
|
|
|
cisternae
|
folds of golgi bodies
|
|
|
Contractile vacuole
|
has a small pore that opens to the outside of the cell and pumps accumulated water out this pore.
|
|
|
oxidative metabolism
|
chemical reaction takes place in mitochondria by extracting energy from organic molecules.
|
|
|
endosymbiosis
|
eukaryotic cells evolved by symbiosis of a prokaryotic species. Chloroplasts / mitochondria. Evidence. both have two membranes. same size as bacteria similar to bacteria's ribosomes circular molecules of DNA and mitochondria replicate bysimple fission
|
|
|
Cytoskeleton
|
microtubules, intermediate filaments and actin filaments. Plays role in determining shape of animal cell. Provides scaffold for ribosomes to carry out synthesis and enzymes to be localized.
|
|
|
Cell movement
|
cells can crawl.
microtubular structure for cilia and flagellum : basal body. |
|
|
aquaporins
|
small pores that allow water molecules to traverse the nuclear membrane.
|
|
|
Facilitated diffusion
|
selective transport across a membrane in the direction of lower concentration
|
|
|
cholestrol
|
is a cyclic hydrocarbon which is not considered saturated nor unsaturated.
|
|
|
Chemiosmosis
|
process of atp formation
|
|
|
Phosphorylation
|
the process of transferring a phosphate group from a donor to an acceptor; often catalysed by enzymes
|
|
|
Substrate level phosphorylation
|
transfer of a high energy phosphate group from a substrate of ADP
|
|
|
Oxidation
|
when an atom or molecule loses an electron in a chemical reaction ( usually hydrogen )
|
|
|
reduction
|
when an atom gains an electron it is reduced.
|
|
|
atomic number
|
atoms with same atomic number are said to exhibit the same chemical properties and are said to be considered the same element.
|
|
|
radioactive decay
|
some isotopes are unstable and break up into particles with lower atomic numbers. Dating fossils. Medical Procedures.
|
|
|
van der waals forces
|
weak kind of chemical attraction when atoms are very close to each other
|
|
|
amino group
|
carboxyl group
functional group an amino group |
|
|
5 factors promoting protein structure forming
|
1. disulfide bridges.
2. hydrogen bonds. 3. van der waals forces. 4. ionic bonds 5. hydrophobic effects |
|
|
nucletoide
|
phosphate molecule
5 carbon sugar nitrogeneous base |
|
|
carbohydrate
|
any molecule carbon:Hydrogen:oxygen 1:2:1
|
|
|
Fats
|
3 fatty acid
1 glycerol C H chains |
|
|
steroids
|
four interconnected rings of carbon atoms. not soluble. cholestrol and estrogen
|
|
|
phospholipids
|
FA, phosphate, glycerol
|
|
|
Pilus
|
aids in exchanging genetic information between cells
|
|
|
centrioles
|
only animal cells
|
|
|
cholesterol
|
found in between phospholipid bilayer it affects fluid nature of membrane, accumulates and can cause plaques, these plaques lead to cardiovascular disease.
|
|
|
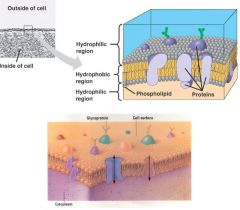
transmembrane proteins
|
form channels that span membrane to move substances in and out of cells
|
|
|
cell surface proteins
|
are attached to the outer surface of the membrane and act as markers. receive and transmit messages to cell once triggered by specific hormone or protein. Allows recognition of self.
|

