![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Describe the De Broglie relationship. |
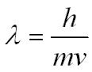
This relationship gives rise to the quantisation of energy. A continuous wave in a confined space can annihilate itself through destructive interference, unless the frequencies are selected carefully. |
|
|
Describe what quantum numbers n, l, ml and ms represent. |
n - energy of the electron. l - shape of orbital ml - orientation of orbital ms - electron spin |
|
|
What is the Aufbau Principle? |
The electronic configuration is built up by putting electrons in the lowest energy orbital first. |
|
|
What is Hund's rule? |
Electrons occupy orbitals of similar energy singly before spin pairing occur. |
|
|
What is Pauli Exclusion? |
For every orbital there can be a maximum of 2 electrons. |
|
|
Why is Na electropositive? |
Na has one electron in its outer shell that is shielded from 11 protons by 10 electrons. It is further away and at high energy and can therefore lose electrons easily to achieve nobel gas configuration. It is held very tightly and has a higher tendency to take in another electron to achieve nobel gas configuration. |
|
|
Why is F electronegative? |
F has 7 electrons in its outer shell that is shielded from 9 protons by 2 electrons. It is further away and at high energy and can therefore lose electrons easily to achieve nobel gas configuration. |
|
|
Describe the bond between sodium and chlorine. |
Ionic bond. Na would give its electron to Cl and form a 3D stable lattice. |
|
|
Describe the F-F bond. |
Covalent bond. 2 electronegative atoms achieve octet rule by sharing electrons. |
|
|
What can't carbon-carbon bonds in an ethene or ethyne rotate? |
The p-orbitals do not overlap properly sine the c-c sigma bond is already overlapping. If the c-c bond rotate, this would break the p orbital. |

