![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
101 Cards in this Set
- Front
- Back
|
cytoskeletal filaments? |
1. microfilaments 2. microtubules 3. intermediate filaments 4. regulated assembly from a large number of locations 5. highly dynamic 6. polarized 7. tracks for myosin 8. contractile machinery and network at the cell cortex |
|
|
microfilaments |
1. actin binds ATP 2. form rigid gels, networks, and linear bundles 3. regulated assembly from a large number of locations 4. highly dynamic 5. polarized 6. tracks for kinesins and dyneins 7. organization and long-range transportation of organelles |
|
|
microtubules |
1. alpha-beta tubulin binds GTP 2. rigid and not easily bent 3. assembled onto pre-existing filaments 4. less dynamic 5. unpolarized 6. no motors 7. cell and tissue integrity 8. assemble and radiate from specific sites 9. extend and probe the edge of the cell |
|
|
intermediate filaments |
1. IF subunits don't bind a nucleotide 2. great tensile strength 3. non polarized fibrous component of the cytoskeleton 4. do not have associated motor proteins 5. build from coiled-coil dimers 6. associate into an antiparallel fashion into tetramers and then protofilaments (16 make a filament) 7. associated with defects in intermediate filaments (esp. laminopathies and keratin gene mutations) |
|
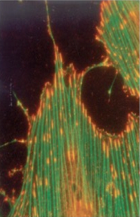
|
microfilaments |
|
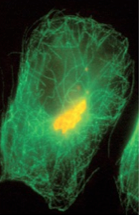
|
microtubules |
|
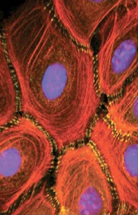
|
intermediate filaments |
|
|
where are microtubules found? |
1. cytoplasmic networks 2. mitotic spindle 3. axons 4. cilia and flagella |
|
|
how are microtubules formed? |
1. protofilaments form a tubule 2. composed of tubulin protein 3. numerous MAPs 4. built from tubulin dimers |
|
|
protofilaments |
1. form tubule 2. 13 repeating units 3. two headed kinesin-1 binds beta-subunits every 8mm along protofilaments 4. tetramers assemble into protofilaments 5. wrap around one another to form 10 nm filaments 6. 16 protofilaments make up a filament
|
|
|
MAPs |
1. side-binding microtubule-associated proteins 2. found in microtubule formation 3. promotes MT assembly 4. stabilize microtubules 5. also called +TIPs 6. bind selectively to growing (+) ends of microtubules 7. can alter the dynamic properties of the microtubule 8. can localize components to the searching (+) en do the microtubule
|
|
|
tubulin dimers |
1. one alpha- and one beta-tubulin subunit 2. build microtubules |
|
|
alpha and beta tubulin |
1. closely related subunits 2. 55 kD 3. binds GTP at interface with the beta-subunit 4. alpha/beta tubulin monomers assemble into filaments with specific structure and polarity 5. assemble into microtubules having 13 laterally associated protofilametns |
|
|
gamma tubulin |
1. serves a regulatory functions 2. part of pericentriolar material 3. forms ring complex (gamma-TURC) which may nucleate MTs in the centriole |
|
|
tubulin |
1. binds GTP 2. subunits binds GTP 3. major structural component of microtubules 4. associate with MAPs
|
|
|
alpha tubulin subunit |
1. binds a trapped and nonhydrolyzable GTP 2. binds GTP at interface with the beta-subunit 3. unable to hydrolyze GTP 4. exposed at the (-) end |
|
|
beta-tubulin subunit |
1. slowly hydrolyzes GTP 2. binds an exchangeable and hydrolyzable GTP 3. located at the (+) end |
|
|
microtubule polarity |
1. alpha/beta tubulin monomers assemble into filaments with specific structure and polarity 2. the "+" end is favored for assembly |
|
|
single microtubules |
have 13 protofilaments |
|
|
doublet microtubules |
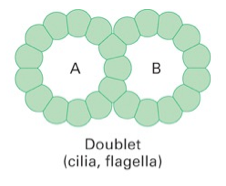
consist: 1. A tubule with 13 protofilaments 2. B tubule with 10 protofilaments |
|
|
triplet microtubules |
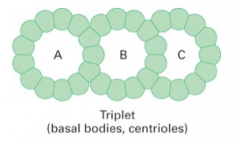
consist: 1. A tubule with 13 protofilaments 2. B tubule with 10 protofilaments 3. C tubule with 10 protofilaments |
|
|
what is one way to show organization in cells? |
immunofluorescence |
|
|
microtubule organization in cells |
1. assemble and radiate from specific sites 2. extend and probe the edge of the cell 3. MT nucleation occurs at MTOCs 4. minus (-) ends are anchored in the MTOCs 5. plus (+) ends are found pointing out to the cell periphery
|
|
|
MT nucleation |
1. spontaneous nucleation is rare in vivo 2. occurs at microtubules-organizing centers (MTOCs)
|
|
|
MTOC |
1. where MT nucleation occurs 2. minus ends of microtubules are anchored to MTOC 3. usually close to the nuclease (during interphase) 4. microtubule-organizing centers 5. ex: centrosome |
|
|
centrioles |
1. pair of orthogonally arranged cylindrical MT structures with a pericentriolar 2. 9 triplet microtubules 3. associate with the base of a cilia and flagella (basal bodies)
|
|
|
pericentriolar material |
1. amorphous material associated with MT structures 2. gamma tubulin is a part of the pericentriolar marterial |
|
|
basal bodies |
1. centrioles associated with the base of cilia and flagella 2. MTOCS that assemble cilia and flagella 3. structures with nine sets of outer triplet microtubules 4. closely related to microtubules |
|
|
microtubule dynamics |
1. MT assembly is promoted by MAPs 2. cycle of assembly and disassembly enriches MAPs that promote MT dynamics 3. treadmilling can occur |
|
|
elongation |
rapid |
|
|
steady state |
when assembly is balanced by disassembly |
|
|
why can treadmilling occur? |
because there are different critical concentrations at the different ends of MTs |
|
|
intracellular tubulin concentration |
much higher than the critical concentration for assembly |
|
|
dynamic instability |
1. cycles of MT assembly and then catastrophic disassembly 2. individual microtubule (+) ends undergo dynamic instability 3. alternating periods of growth or rapid shrinkage 4. microtubules can search the cytoplasm for structures or organelles with appropriate targets and capture them --> stabilization of the (+) |
|
|
GDP-beta-tubulin |
1. curved 2. protofilaments lose lateral interactions 3. splay and disassemble |
|
|
GTP-beta-tubulin |
1. strong lateral interactions between straight protofilaments 2. added during assembly |
|
|
what happens when there is a lag in assembly? |
1. GTP hydrolysis occurs at MT ends 2. produces catastrophe |
|
|
what regulates cellular MT distribution and functions? |
MAPs |
|
|
Tau family |
1. Tau, MAP2, MAP4, etc 2. bind to MT walls 3. stabilize MTs 4. crossbridge MTs in axons |
|
|
+TIP proteins |
1. EB1 2. bind and stabilize + ends of microtubules 3. associate with other proteins and organelles to redistribute these cargo via microtubule polymerization |
|
|
kinesin-13 family |
1. not a motor protein 2. binds curved protofilaments at GDP-tubulin MT + ends 3. dissociating tubulin dimers and enhances catastrophe frequency 4. can destabilize microtubule ends |
|
|
Op18/stratmin |
1. overexpressed in some cancers 2. binds curved protofilaments at GDP-tubulin MT +ends 3. can destabilize microtubules 4. enhances catastrophe frequency
|
|
|
MT motors |
1. kinesins and dyneins 2. transport organelles and other cargo long distances (like in axons) 3. movements can occur in response to signaling pathways, like in pigment cells |
|
|
axonal transport |
1. cargo movement necessary in NS to get proteins synthesized in soma to synaptic terminals 2. fast movement (250 mm/day) = 4 days to travel from soma to terminals in toe |
|
|
anterograde movement |
cell body to synaptic terminals |
|
|
retrograde movement |
from terminals to cell body |
|
|
Kinesin-1 |
1. motor protein 2. (+) end directed 3. responsible for anterograde axonal transport 4. two heavy chains each with: N terminal motor domain, two light chains associated with cargo 5. has a pair of globular head domains, short flexible linker domain to a long central stalk, pair of globular tail domains that associate with light chains 6. organelle associated 7. ATP dependent 8. transports membrane-bound organelles 9. highly processive motor 10. coordinates ATP hydrolysis between its two heads so that one is always firmly bound to a microtubule |
|
|
kinesin-2 |
1. organelle associated 2. transport cargo |
|
|
transport cargo kinesins |
kinesin 1 and kinesin 2 |
|
|
organelle associated kinesins |
kinesin 1 and kinesin 2 |
|
|
kinesin-5 |
1. bipolar 2. produces sliding of microtubules against one another in mitosis |
|
|
kinesin-1 motor movement |
1. two headed kinesin-1 binds beta-subunits every 8mm along a protofilament 2. each step by one head domain takes 16nm steps 3. starting with the leading head of kinesin in a nucleotide-free state 4. strongly binds beta-tubulin 5. trailing head has bound ADP 6. ATP binding by forward head induces forward motion of linker 7. swinging the lagging head forward 8. new leading head binds beta-tubulin 9. releases ADP --> induces ATP hydrolysis in the other head 10. ATP hydrolysis and inorganic phosphate release converts kinesin head into the weakly bound state 11. leading head is now ready to bind ATP 12. cycle repeats |
|
|
dynein |
1. minus end directed motors 2. used to transport vesicles and organelles via retrograde movement 3. large, multi subunit complexes 4. less diverse family than kinesins and myosins 5. two headed molecules with MT binding (stalk), ATPase (head) and dynactin binding (stem) domains 6. power stroke involved in rotation of round head domain |
|
|
dynein stalk |
MT binding |
|
|
dynein head |
ATPase |
|
|
dynein stem |
dynactin stem |
|
|
dynein power stroke |
rotation of the round head domain |
|
|
what does dynein transport require? |
dynactin |
|
|
dynactin |
1. used in dynein transport 2. mediates cargo movements 3. multi-subunit complex 4. connects dynein to cargo |
|
|
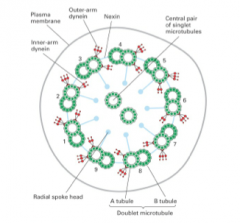
cilia and flagella |
1. cellular processes on the cell surface that extend a few um to a 2 mm 2. contains axonemes 3. microtubule based cell-surface structures 4. characteristic central pair of singlet microtubules and nine sets of outer doublet microtubules 5. grow from basal bodies |
|
|
axoneme |
1. central bundle of microtubules 2. arranged in a 9+2 arragnement 3. motility is produced by regulated activation of axonemal dynein motors |
|
|
dynein motor structure |
1. associated with the A-tubule of the outer doublet 2. bind the B-tubule during bending |
|
|
nexin linkages |
between doublet microtubules cause bending of axonemes |
|
|
what caused the bending of axonemes |
nexin linkages |
|
|
intraflagellar transport |
1. components required for flagellar assembly 2. maintenance are transported over the outer surface of the axoneme |
|
|
ciliopathies |
ADPKD, retinal degeneration, olfaction defects |
|
|
10 nm filaments |
intermediate filaments |
|
|
IF assembly |
1. parallel dimers formed by coiled-coil motifs 2. antiparallel tetramers are the monomer assembly subunit for filament |
|
|
epidermolysis bullosa simplex |
1. blistering of epidermis 2. knockout of mouse K14 causes this disorder 3. caused by mutations in keratin genes |
|
|
microtubule cell walls |
polarized structures built from alpha-beta tubulin dimers |
|
|
centrosome |
1. surrounded by pericentriolar matrial 2. type of MTOC 3. nucleates the radial array of microtubules in non-mitotic animals 4. consist of two centrioles and the pericentriolar material that contains gamma-TuRC microtubules-nucleating complex
|
|
|
free tubulin |
exists as an alpha-beta dimer |
|
|
beta-tubulin |
1. bound to GDP 2. (+) ends capped by GTP-beta-tubulin 3. (+) ends blunt or slightly splayed out |
|
|
shrinking microtubules |
1. have lost the GTP-beta-tubulin cap 2. caused the protofilaments to peel outward and dissassemble |
|
|
growing microtubules |
1. store the energy derived from GTP hydrolysis in the microtubule lattice 2. they have the potential to do work while diassassembling |
|
|
"search and capture" |
1. contrubutes to the overall distribution of microtubules in a cell 2. microtubules from centrosomes and have dynamic instability 3. microtubules "search" the cytoplasm for structures or organelles with appropriate targets and "capture" them 4. stabilizes the (+) end of the microtubule |
|
|
kinesin superfamily |
1. includes motors that function in interphase and mitotic cells 2. transport organelles 3. sliding antiparalllel microtubules past one another 4. includes at least one class, kinesin-13, that is not motile but destabilizes microtubule ends |
|
|
cytoplasmic dyenin |
1. microtubule (-) end directed motor 2. ATP dependent 3. associates with the dynactin complex to transport cargo |
|
|
kinesins and dyneins |
associate with many different organelles to organize their location in cells |
|
|
tubulin post-translational modifications |
can affect microtubule stability and regulate their ability to interact with microtubule-based motors |
|
|
five major classes of intermediate filament proteins |
I: Acid keratins II: Basic keratins III: Desmin, GFAP, vimentin IV: Neurofilaments V: Lamins |
|
|
Class I intermediate filament proteins |
1. Acid keratins 2. show tissue-specific expression |
|
|
Class II intermediate filament proteins |
1. basic keratins 2. show tissue-specific expression |
|
|
Class III intermediate filament proteins |
1. Desmin, GFAP, vimentin 2. show tissue-specific expression 3. provide structure and order to muscle Z disks 4. restrain smooth muscle from overextension |
|
|
Class IV intermediate filament proteins |
1. neurofilaments 2. show tissue-specific expression 3. important for structure of axons |
|
|
Class V intermediate filament proteins |
1. nuclear lamins 2. most ancient and ubiquitous in animal cells
|
|
|
Keratins (class I and II) |
1. found in animal hair, nails, and cytokeratin filaments 2. associate with desmosomes in epithelial cells 4. provide the cells and tissues with strength |
|
|
laminopathies |
1. defects in intermediate filaments 2. include a variety of conditions |
|
|
mutations in keratin genes |
1. defects in intermediate filaments 2. can cause severe defects in skin |
|
1. light green 2. dark green 3. line I 4. line 2 |
1. alpha tubulin 2. beta tubulin 3. GTP 4. GDP |
|
|
what end of the microtubule is favored for polarity? |
the plus end |
|
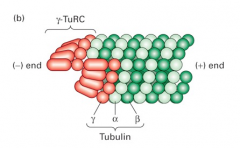
|
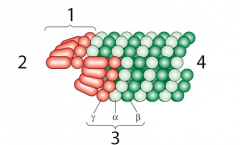
pericentriolar material |
|
|
1. gamma-TuRC 2. (-) end 3. Tubulin 4. (+) end |
|
|
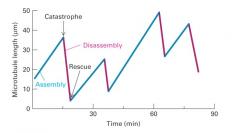
dynamic instability |
|
|
1. assembly 2. catastrophe 3. disassembly 4. rescue |
|
|
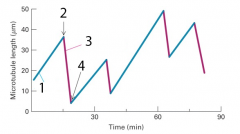
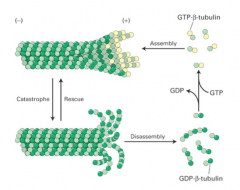
dynamic instability |
|
|
dynamic instability |
|
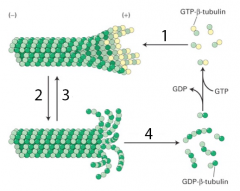
|
1. assembly 2. catastrophe 3. rescue 4. diassembly |
|
|
dynamic instability mechanism |
1. GTP beta-tubulin binds to (+) end of microfilament 2. catastrophe happens 3. (+) disassembles into GDP beta-tubulin 4. after disassembly, rescue occurs 5. rescue leads to normal assembled microfilament |
|
|
kinesin-1 motor movement |
1. leading head binds to ATP 2. binding ATP induces a conformational change causing neck linker to swing forward and dock into head 3. motion swings the former trailing head to become the leading head 4. new leading head finds a binding site on the microtubule 16nm ahead of its previous site 5. leading head release ADP 6. trailing head hydrolyzes ATP to ADP + Pi 7. Pi is released 8. linker becomes undocked |
|
|
cilia/flagella structure |
|

