![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
|
contains 3 carbon atoms, e.g., glyceraldehyde
|
triose
|
|
|
Meth-
|
1
|
|
|
Eth-
|
2
|
|
|
Prop-
|
3
|
|
|
But-
|
4
|
|
|
Pent-
|
5
|
|
|
-ane
|
Alkanes
|
|
|
-ene
|
Alkenes
|
|
|
-yne
|
Alkynes
|
|
|
Hex-
|
6
|
|
|
Hept-
|
7
|
|
|
Oct-
|
8
|
|
|
Non-
|
9
|
|
|
Dec-
|
10
|
|
|
also called saturated hydrocarbons
|
alkanes
|
|
|
the longer the carbon chain, the higher the _______ _____
|
boiling point
|
|
|
R - X or Ar - X
|
Organic Halides
|
|
|
R-OH
|
Alcohols
|
|
|
Ar-OH
|
Phenols
|
|
|
R-O-R'
|
Ethers
|
|
|
R-NH2 or Ar-NH2
|
Amines
|
|
|
Hydrocarbon with triple bonds
|
alkyne
|
|
|
Hydrocarbon with double bonds
|
alkene
|
|
|
Hydrocarbon with single bonds
|
alkane
|
|
|
RCONH2
|
Amides
|
|
|
RCOOR'
|
Esters
|
|
|
R-COOH or Ar-COOH
|
Carboxylic acids
|
|
|
R-COR'
|
Ketones
|
|
|
R-CHO or Ar-CHO
|
Aldehydes
|
|
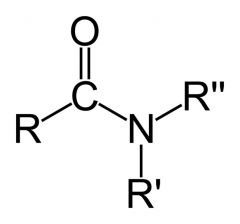
_____ functional group
|
Amide
|
|
|
characterized by their pleasant fruity odors
|
Esters
|
|
|
An _____ is named according to the two parts that make it up: the part from the alcohol and the part from the acid (in that order), for example ethyl ethanoate.
|
ester
|
|
|
have a wide range of household uses
|
carboxylic acids
|
|
|
vinegar or acetic acid is an example of a __________ _____
|
carboxylic acids
|
|
|
__________ _____ with long carbon chains are usually solids at room temperature. They are called fatty acids and they may be saturated or unsaturated.
|
carboxylic acids
|
|
|
In IUPAC nomenclature, __________ _____ have an -oic acid suffix (e.g., octadecanoic acid). In common nomenclature, the suffix is usually -ic acid (e.g., stearic acid).
|
carboxylic acids
|
|
|
In general, _______ are named using IUPAC nomenclature by changing the suffix -e of the parent alkane to -one. For common _______, some traditional names such as acetone and benzophenone predominate, and these are considered retained IUPAC names [2], although some introductory chemistry texts use names such as propanone.
|
ketones
|
|
|
_________ and _______ usually have an appealing taste and a fragrant odor.
|
Aldehydes; ketones
|
|
|
The simplest aldehyde is used as a "pickling" solution for biological specimens.
|
formaldehyde or formalin
|
|
|
the simplest ketone is used for dissolving fatty substances
|
Acetone
|
|
|
The name is formed by changing the suffix -e of the parent alkane to -al, so that HCHO is named methanal, and CH3CH2CH2CHO is named butanal.
|
aldehydes
|

