![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
CTP is able to influence the activity of ATCase. This is an example of: |
Inhibition by an eventual product of the enzymatic pathway |
|
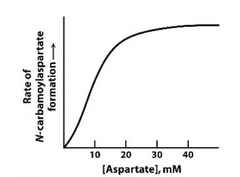
Part 1: This is a profile of the ATCase (Aspartate transcarbamolyase). What does this profile indicate? |
Cooperativity |
|
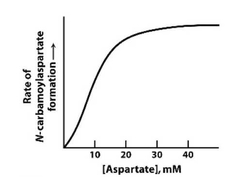
Part 2: How is this different from allosteric substrate stimulation. |
Allosteric stimulation involves a second site, whereas with ATCase the substrate stimulation targets the active site. This is an example of unexpected kinetics as the result of a complex multi-protein complex. |
|
|
The regulatory subunits of ATCase: |

|
|
|
If you were to find a molecule that could mimic the binding of CTP to the ATCase enzyme in an irreversible way, what would the result be? |
ATP would no longer be an inducer |
|
|
In the T state, ATCase: |
Binds substrate which promotes transition to the R state. |
|
|
You have isolated the inhibitory subunit of ATCase. What would the activity be? |
It would bind ATP |
|
|
The regulatory subunit of PKA: |
Binds as a pseudosubstrate to the active enzyme |
|
|
What is an isozyme. |
Distinct enzymes that have the same catalytic functions. |
|
|
What is the nature of inhibition of protein kinase A by its regulatory subunit? |
Competitive inhibition |
|
|
How does cAMP overcome this inhibition? |
By inducing a conformational change through direct binding to the enzyme |
|
|
How is a G-protein normally inactivated? |
By hydrolysis of GTP |
|
|
The ATCase enzyme is designed to: |
Increase in activity within a narrow range of substrate concentrations |
|
|
In the ATCase enzyme complex, CTP binds: |
To the regulatory subunit only |
|
|
In ATCase, there are multiple catalytic subunits. How do they associate together? |
They form two separate trimers |
|
|
Cooperativity in an enzyme indicates: |
That separate enzyme subunits in a complex influence one another. |
|
|
Why does ATP stimulate ATCase? |
Because if ATP is able to stimulate it must be in concentration excess over CTP. It is in the interest of the cell to keep these two molecules in general equilibrium because both are used in near equal proportions during nucleotide biosynthesis. |
|
|
If the concentration of CTP increases, what is the effect upon ATCase activity? |
It induces inhibition which can be overcome by substrate |
|
|
Tyrosine kinases tend to be involved in: |
Growth control |
|
|
The regulatory subunit of PKA |
Binds as a pseudosubstrate to the active enzyme |
|
|
Cyclic AMP |

|
|
|
Histones are commonly modified by: |
Acetylation |
|
|
What is unique about different types of G-protein coupled receptors (G-protein receptors) compared to other types of cell surface receptors. |
They have seven transmembrane regions. |
|
|
When epinephrine binds its receptor: |

|
|
|
When bound to GTP the G alpha subunit: |
Hydrolyzes GTP to become inactivated. |
|
|
G alpha DIRECTLY does which of the following: |
Promotes formation of cAMP |
|
|
What would happen if GTP hydrolysis by G proteins were eliminated? |
They would remain continuously active. |
|
|
Cyclic AMP does which of the following? |

|
|
|
Tyrosine kinases: |

|
|
|
When bound to EGF, the EGF receptor: |

|
|
|
When bound to EGF the EGF receptor |
Initiates a cascade that results in cell growth |
|
|
If the ability of Ras to hydrolyze GTP were reduced, what would most often happen? |
Uncontrolled cell proliferation |
|
|
According to our discussion in class, why do you think CTP should not be able to completely inhibit ATCase? (Be brief!) |
A healthy cell needs CTP constantly for mRNA synthesis. It is constantly needed and should constantly be synthesized. The rate of synthesis should be regulated, not the overall synthesis itself. |
|
|
Why are mutations that reduce the GTPase activity of Ras often seen in human tumors? |
GTPase activity naturally limits the activity of Ras. Without this activity the signaling of Ras would be excessive, commonly participating in tumor formation. |
|
|
Why should ATP stimulate ATCase activity? Where does it bind? |
It binds to the regulatory site in the regulatory subunit, the same site to which CTP binds. ATP stimulates in order to: 1) Keep the concentration of CTP near that of ATP, since both are used in equivalent proportions for nucleotide synthesis. 2) Because high ATP concentration indicates that there is sufficient energy for the cell to continue active metabolism, which would require CTP. |

