![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
ATP (Adenosine 5-triphosphate)
|
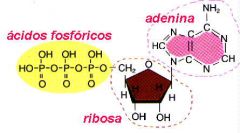
What is the name of this molecule?
|
|
|
What is the most impotant nucleotide? Why?
|
Adenine; ATP the energy currency of all life.
|
|
|
Adenine
|
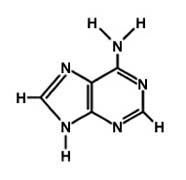
What is the name of this molecule?
|
|
|
Guanine
|
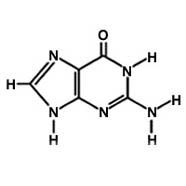
What is the name of this molecule?
|
|
|
Cytosine
|
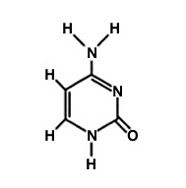
What is the name of this molecule?
|
|
|
Thymine
|
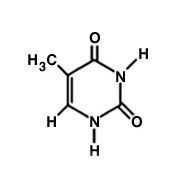
What is the name of this molecule?
|
|
|
Uracil
|
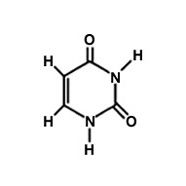
What is the name of this molecule?
|
|
|
What is the difference between ribose and deoxyribose?
|
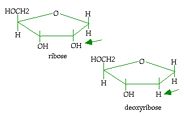
see picture
|
|
|
Sugar + Base=
|
Nucleoside
|
|
|
Nucleoside (sugar+base) + phosphate group=
|
Nucleotide
|
|
|
Which phosphate of the tri phosphate on ATP is most acidic? least acidic?
|
One closest to the Adenosine (pka-2); the one furthest from the adenosine (pka-7). the other has a pka of 4.
|
|
|
what is the net charge on a molecule of ATP? What is the significance of this?
|
-3.5; the signinficance is that this is the reason that ATP has so much energy stored in its bonds. It is so unstable it yields alot of energy in the breaking of its bonds.
|
|
|
Why is ATP measured with an ASSOCIATION constant rather than a dissociation constant?
|
Because we use ASSOCIATION constants for any gaseous molecule and metal complexes. ATP forms a TIGHT complex with Mg2+. (pH dependent)
|
|
|
What is the role of Adenylate kinase? where is it found?
|
Adenylate kinase catylizes the reaction of MgADP to MgATP by using AMP.
Found in between the 2 mitochondrial membranes, its role is to mantain balance of ATP : ADP+AMP in the body. |
|
|
The ATP cycle describes...
|
ATP being expended to do work (motion, active transport, biosynthesis) and formed by oxidation of fuels, photosynthesis of dephosphorylated ATP such as AMP and ADP.
|
|
|
How are ATP levels in the muscle regulated differently than those in the body?
|
In the muscle cells phosphates are stored in the form of PHOSPHOcreatin and the enzyme creatin kinase breaks the high energy P-N bond when energy is needed.
|
|
|
Finish the overall reaction:
Phosphocreatin + MgADP ---> (enzyme?) |
Creatin + mgATP
(creatin kinase) |
|
|
For the creatin/phosphocreatin reaction in muscle cells, what is the Keq of the reaction and what does it indicate?
|
50; The reaction favors the formation of MgATP and Creatin.
|
|
|
What are the three differences and one similarity between Combustion reactions and metabolic reactions?
|
1.) No enzymes in combustion, many in metabolitic pathways.
2.) No distinct intermediates in combustion. 3.) Regulation is difficult in combustion and easy in metabolitic. Similarities: the Gibbs free energy and STANDARD STATE Gibbs free energy are the same in both. |
|
|
Give the overall reaction for the metabolitic combustion of glucose. What is the gibbs free energy for this process and what does it mean?
|
Glucose+Water <---> 6 CO2 + 6 H20
Gibbs free energy is -2.87e3 kJ/mol. the negative means that the reaction occurs spontaneously and as we know it is exothermic (delta H). |
|
|
When specifically using
G=H-TS will a reaction occur spontaneously? |
1.) when H is negative and S is positive.
2.) High temperatures even if H is positive and S is positive. 3.) @ low temps when |H|>|S| |
|
|
What does delta G0 prime mean?
|
the delta g at the biochemical standard state...
pH=7 and 1 mol |
|
|
The list in the notes on pg 51 shows all Phosphate compounds capable of transfer reactions.
1.) What do we notice about all of them? 2.) Where is ATP? 3.) True/False molecules with more negative delta g's pass phosphates to those with less negative delta g's? |
1.) All are negative
2.) right in the middle 3.) True. |
|
|
3 reasons why ATP is so exergonic (indicate which is the best explanation).
|
1.) A standard charge of -3.5 makes ATP highly unstable. removing a phosphate (with one -) makes the molecule much more stable and releases alot of energy. (best argument)
2.) Increases the degrees of hydration (?) 3.) Resonance stability of the phosphate group (good leaving group) |
|
|
Why is a carbohydrate called a carbohydrate?
|
because it is a fully hydrated carboxyl group. In other words POLYHYDROXYLATED aldehydes and ketones.
|
|
|
Sugars can be classified by their original carbonyl as being ______ or _____. Then they can be further classified by the number of carbons such as _____ and ____.
|
aldoses and ketoses; triose, tetroses, pentoses
|
|
|
What is the simplest sugar? what is its n=?
|
N=3. N=1 and N=2 are not real sugars.
N=3 is a glyceraldehyde. |
|
|
What do we know about the stereochemistry of all sugars in teh human body?
|
they are in the D form. meaning they rotate light to the right.
|
|
|
How do we add carbon groups to existing sugars to make longer sugar chains? (Hint: Organic synthesis)
|
By reacting the sugar with CN which adds and then react it with CO2.
|
|
|
Adding a carbon group to a glyceraldehyde gives what product.
|
3 carbon sugar ---> 4 carbon
Glyceraldehyde---> D-erythrose and D-threose (diasteromers) |
|
|
What are the 2 IMPORTANT products of a ALDOPENTOSE?
|
D-Ribose and D- arabinose.
|
|
|
What is the following molecule?
CHO H__|__ OH H__|__ OH H__|__ OH | CH2OH |
D-Ribose
|
|
|
What is the name of the following molecule?
CHO OH__|__ H H__|__ OH H__|__ OH | CH2OH |
D-Arabanose
|
|
|
Starting with the 3 carbon aldose name all the diasteromer products of the aldose tree (from triose to hexoses).
|
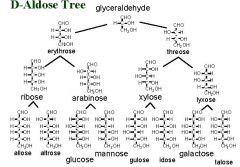
Phewww!
|
|
|
If ribose is given below, what changes are made to make deoxyribose?
CHO H__|__ OH H__|__ OH H__|__ OH | CH2OH |
CHO
H__|__ H H__|__ OH H__|__ OH | CH2OH |
|
|
Why is D-arabinose (5 carbon aldose) so important?
|
It is the derivative of glucose (6 carbon sugar)
|
|
|
What is the molecule below?
CHO H__|__ OH OH__|__ H H__|__ OH H__|__ OH | CH2OH |
D-Glucose
|
|
|
What is the following molecule?
CHO OH__|__ H OH__|__ H H__|__ OH H__|__ OH | CH2OH |
D-Mannose
|
|
|
What is a defenition of an epimer? Give the most well knwon example?
|
Diasteromers that differ in the stereochemistry of 1 carbon. Glucose and Mannose.
|
|
|
What is the simplest ketone?
|
Dihydoxyacetone.
CH20H | C=0 | CH2OH |
|
|
Lengthening the chain to a tetraketose we have what product? Why only one?
|
D-Erythulose; because dihydroxyacetone had no chiral centers and therefore no diastereomers.
CH2OH | C=O |__ | CH2OH |
|
|
Lengthening the chain to a pentaketose we get two products.
|
D-Ribulose and D-Xylulose
CH2OH | C=O |__ |__ | CH2OH and CH2OH | C=O __| |__ | CH2OH |
|
|
The only important hexaketose is... and its structure is...
|
D-Fructose
CH2OH | C=O __| |__ |__ | CH2OH |
|
|
What is the structure of a hemiacetal?
|
OH
| R-C-H | OR' |
|
|
What is the structure of a hemiketal?
|
OH
| R'-C-R'' | OR |
|
|
Why is glucose in a ring form?
|
Because through an SN2 reaction, the oxygen of Carbon # 5 can attack the carbonyl creating a 6 membered ring.
Realize now that the attack can be from below or above and thus two diasteromers result from the cyclization of glucose we call them alpha and beta D-glucose. |
|
|
Which of the diasteromer products of the cyclization of glucose is more stable? WHy?
|
The Betta D-glucose is more stable than the alpha because it has the larger OH (than H) group in the UP POSITION (in chair conformation that would make this position equitorial).
|
|
|
Where is the anomeric carbon of a carbohydrate?
|
That is where the CH2OH group resides.
|

