![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
199 Cards in this Set
- Front
- Back
|
1. After glycolysis and the citric acid cycle how many high energy molecules and electron carriers have we made?
|
2 ATP
2 GTP 2 NADH 2 FADH₂ |
|
|
2. How can we convert all of the NADH and FADH₂ into ATP?
|
Oxidative Phosphorylation
|
|
|
3. What are the four components of the mitochondria?
|
1. Outer membrane (relatively permeable)
2. Inner membrane (site of oxidative phosphorylation) 3. Intermembrane space (stores proteins) 4. Matrix (site of PDH, citric acid cycle, and fatty acid oxidation, where NADH is made) |
|
|
4. What is oxidative phosphorylation?
|
-Oxidative phosphorylation couples the flow of electrons from one acceptor to the next to the translocation of protons across the inner membrane (matrix to inner mitochondrial space)
-The energy stored in this proton gradient is used to make ATP Oxidize NADH and FADHS and donate e- to O2 which is reduced to H2O |
|
|
5. What new electron carriers are used in oxidative phosphorylation?
Three of them... |
1. Cytochromes
2. Fe/S proteins 3. Ubiquinone |
|
|
6. Describe the Fe/S protein carriers?
Five points... What complex is it part of? |
1. One electron carrier
2. Non-mobile 3. Speed transfer of e- between complexes 4. Donates e- to coenzyme Q 5. Pumps about 4 protons and produces 1 ATP Complex I |
|
|
7. Describe cytochromes?
Two points... |
1. Single electron carrier protein
2. Have iron heme prosthetic group |
|
|
8. What role does the heme group play?
|
Fe3+ of heme is reduce to Fe2+ as it accepts electrons
Oxidized back to Fe3+ as it passes electrons down chain 2 states: Oxidized Fe3+ Reduced Fe2+ |
|
|
9. Describe absorption by cytochromes?
|
-Cytochromes absorb light differently depending upon if they are oxidized or reduced
-Due to this property one can follow the path of the electrons through oxidative phosphorylation |
|
|
10. Describe Ubiquinone.
Six chunks... |
1. Small molecule
2. Can carry either one or two electrons 3. Has a very hydrophobic tail 4. Mobile carrier 5. Usually referred to as "Q" 6. Complete reduction requires two electrons and two protons |
|
|
11. What is the hydrophobic tail on ubiquinone?
What does it do? |
-Isoprenoid tail
-The tail serves as a membrane anchor |
|
|
12. Why is ubiquinone a mobile carrier?
|
It can freely diffuse within the lipid bilayer of the inner mitochondria b/c it is a small, hydrophobic molecule
|
|
|
13. How does ubiquinone move electrons?
Three shings... |
1. Gets reduced on matrix side
2. Picks up proton 3. Gets oxidized and releases protons in inner membrane space |
|
|
14. Describe the oxidation states of ubiquinone?
|
-Fully oxidized Ubiquinone ("Q")
-Semiquinone radical ("QH") -Fully reduced Ubiquinol (QH₂) |
|
|
15. How are electron carriers ordered in the electron transport chain?
|
Complex I
Complex II Complex III Complex IV Membrane bound **Transport of 2 e- through each of these complexes is associated with pumping of about 4 H+ from matrix to intermembrane space |
|
|
16. What is each complex?
|
1. Complex I: NADH
dehydrogenase 2. Complex II: Succinate dehydrogenase 3. Complex III: Cytochrome bc1 complex 4. Complex IV: Cytochrome oxidase |
|
|
17. What is one method for determining the order of the electron carriers?
|
-Electrons are passes to electron carriers of increasing affinity (more positive ∆E⁰)
-Measure the ∆E⁰ of specific electrons carriers in the transport chain suggests the order of the pathway -Arrange from least to greatest affinity, where the carrier with the highest affinity receives the electrons last |
|
|
18. What is the problem with the method of measuring ∆E⁰?
|
∆E⁰ doesn't necessarily equal ∆E so need another way to determine order
|
|
|
19. What is another way to determine the order of the electron carriers?
|
-Use specific inhibitors to elucidate the order of the pathway
-In the presence of O₂ and an electron donor, carriers that function before the inhibited step become fully reduced and those that function after this step are completely oxidize |
|
|
20. Where do electrons enter the electron transport chain?
|
-Enter through complex I and II
-Electrons from NADH enter through complex I -Electrons from FADH₂ enter through complex II |
|
|
21. What is complex I called?
|
NADH Dehydrogenase
|
|
|
22. What is complex II called?
|
Succinate Dehydrogenase
|
|
|
23. What two processes does Complex I catalyze simultaneously?
|
1. Exergonic transfer to ubiquinone of a hydride ion from NADH and a proton from the matrix
2. Endergonic transfer of four protons from the matrix to the intermembrane space (pumps protons) |
|
|
24. What is the movement of protons?
|
Protons move from the matrix (which becomes negatively charged) to the intermembrane space (which becomes positively charged)
|
|
|
25. What symbols are used to designate the intermembrane space and the matrix?
Two symbols... |
P for the positive side of the intermembrane space
N for the negative side of the matrix |
|
|
26. Complex I
|
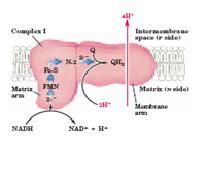
NADH Dehydrogenase
|
|
|
27. What is the path of electrons through complex II?
Two steps... |
1. Electrons move from succinate to FAD
2. From FAD electrons move through three Fe-S centers to ubiquinone |
|
|
28. Does complex II pump protons?
|
NO
|
|
|
29. What is the role of the heme b of complex II?
|
-Not in the direct path of e- transfer
-Serves to reduce the frequency w/ which e- "leak" out of the system moving from succinate to molecular oxygen |
|
|
30. What very bad thing(s) result from electrons moving to molecular oxygen?
|
1. Reactive oxygen species (ROS) hydrogen peroxide (H₂O₂)
2. Superoxide radical (·O₂⁻) |
|
|
31. What other substrates for mitochondrial dehydrogenases pass electrons into the chain at the level of ubiquinone but not through complex II?
|
1. Glycerol 3 phosphate
2. Fatty acyl-CoA |
|
|
32. How does glycerol 3 phosphate contribute electrons?
Three things... |
1. Transports e- into the cell
2. Oxidized by glycerol 3-phosphate dehydrogenase 3. Channels e- into the respiratory chain by reducing ubiquinone (form QH₂) |
|
|
33. How does fatty acyl-CoA contribute electrons?
|
Through the oxidation of fatty acyl-CoA by acyl-CoA dehydrogenase to generate FADH₂
|
|
|
34. What happens to the electrons from the FADH₂ generated by acyl-CoA dehydrogenase?
Three steps... |
1. Electrons are passed sequentially to electron-transferring flavoprotein (ETF)
2. ETF passes e- to ETF:ubiquinone oxidoreductase 3. Finally e- go to Q to make QH₂ |
|
|
35. What does complex III do?
|
Couples the transfer of e- from QH₂ to cytochrome c with the vectorial transport of protons from the matrix to the intermembrane space
|
|
|
36. What does vectorial mean?
|
Moves protons in a specific direction from one location to another
|
|
|
37. Remember what is cytochrome c again?
|
The second diffusible (mobile) electron carrier
*Not part of complex but in between III and IV *single e- carrier transporting e- between cytochrome b-c1 complex and cytochrome oxidase |
|
|
38. Does complex III pump protons?
|
-Yes
-Pumps 4 protons per 2 electrons |
|
|
39. How can one QH₂ pump four H+?
|
Through the Q cycle
|
|
|
40. What exactly does the Q cycle do?
|
1. Accommodates switch between the 2e- carrier ubiquinone and 1e- carrier cytochrome c
2. Explains measured stoichiometry of 4H+ translocated per 2e- |
|
|
41. What is the net effect of the Q cycle's transfer?
|
1. QH₂ is oxidized to Q
2. Two molecules of cytochrome c are reduced |
|
|
42. What is the overall picture of the Q cycle?
Two points... |
1. On the P side, two QH₂ are oxidized to Q releasing two H+ per Q into the N side
2. Each QH₂ donates one e- to cytochrome c and one e- to a molecule of Q near the N side reducing it in two step to QH₂ |
|
|
43. Describe what happens in the first oxidation of QH₂ during the Q cycle.
Three steps... |
1. One e- reduces cytochrome c which goes to complex IV and donates the e-
2. One e- reduces ubiquinone to semi-ubiquinone 3. Two protons are pumped out |
|
|
44. Describe what happens in the oxidation of the second QH₂.
Three steps... |
1. Reduce cytochrome c again
2. Second e- is recycled and stuck onto semi-ubiquinone (reduce it) 3. Release two H+ |
|
|
45. What does complex IV do?
|
Carries e- from cytochrome c to molecular oxygen, reducing it to H₂O
|
|
|
46. Does complex IV pump protons?
|
-YES
-Translocation of 2 more protons |
|
|
47. What is the total number of protons pumped from the electron transport chain if you begin with NADH?
|
Pumps a total of 10 protons
|
|
|
48. What is the total number of protons pumped from the electron transport chain if you begin with FADH₂?
|
Pumps a total of 6 protons
|
|
|
49. Which generates more ATP: NADH or FADH₂?
|
NADH b/c it translocates more protons
|
|
|
50. In what two ways does the proton gradient formed (proton-motive force) store energy?
|
1. A concentration gradient
2. An electrical gradient |
|
|
51. What causes the concentration gradient?
|
Difference in concentration
-More protons in the inner membrane space than the matrix |
|
|
52. What causes the electrical gradient?
|
Separation of charge
-More positive charge in the inner membrane space than the matrix |
|
|
53. In essence what is the gradient since it has a separation of charge?
|
A battery
|
|
|
54. How much energy can the proton gradient store?
|
-When ∆pH = 0.75 & ∆Ψ = 0.2 V, ∆G = 23.56 kJ/mol
-The translocation of each mole of protons generates 23 kJ of energy |
|
|
55. Under the previous conditions, how much energy does the oxidation of each mole of NADH correspond to?
|
Each NADH pumps 10 protons so the oxidation of each mole of NADH corresponds to 235.6 kJ/mol
|
|
|
56. What is the ∆G of hydrolysis for ATP in vivo?
|
Approximately 52 kJ/mol
|
|
|
57. What is the theoretical max that the membrane potential is capable of forming?
|
4.5 mol of ATP per mol of NADH
|
|
|
58. What is the actual number believed to be for the membrane potential?
|
2.5 mol of ATP per mol of NADH
|
|
|
59. What is the point of the electron transport chain?
|
To make the proton gradient
|
|
|
60. What is the chemiosmotic model?
|
The controlled dissipation of the proton gradient through a channel in the inner membrane is indirectly coupled to activity of the ATPase synthase
*this is very different than substrate level phosphorylation seen in glycolysis |
|
|
61. What evidence is there for the chemiosmotic model?
Three points... |
1. Blocking e- transport to O₂ blocks formation of ATP
2. Blocking ATP formation blocks e- transport 3. Electron transport can be uncoupled from ATP formation by making the mitochondrial membrane permeable to protons (uncoupling agents) |
|
|
62. What is an uncoupling agent?
|
A membrane permeable proton carrier the dissipates the proton gradient
-They have a dissociable proton and are very hydrophobic -In essence they short circuit electron transport chain |
|
|
63. What is one uncoupling agent?
|
2,4-Dinitrophenol (DNP)
|
|
|
64. By blocking ATP production e- transport is blocked. Is there any way to correct for this and resume the transport of e-?
|
Electron transport can resume if the proton gradient is dissipated so add DNP
|
|
|
65. In the absence of e- transport ATP production is blocked.
Is there any way to correct for this and have ATP production resume? |
An artificial proton and electrical gradient can drive ATP synthesis
|
|
|
66. What is ATP synthase?
|
A large complex of enzymes that mechanically couples the proton gradient to ATP synthesis
Utilizes energy from gradient to synthesize ATP |
|
|
67. What are the two parts of the ATP synthase?
|
1. The F₁ part (N side)
2. The F₀ part (P side) |
|
|
68. Describe the F₁ part of the ATP synthase?
Three things... |
1. Peripheral membrane protein
2. Soluble ATP synthase 3. The generator |
|
|
69. Describe the F₀ part of the ATP synthase?
Four things... |
1. Integral membrane bound
2. Transmembrane channel 3. The turbine 4. H+ flows in on the p side |
|
|
70. What is the major energy barrier?
|
-In the reaction catalyzed by ATP synthase, release of ATP from the enzyme is the major energy barrier
|
|
|
71. What is the free-energy change for ATP formation?
|
In aqueous solution, the the free-energy change for the formation of ATP is large and positive
|
|
|
72. Since the free-energy change is positive how come ATP formation is reversible with ATP synthase catalyzing?
|
On the enzyme surface, the very tight binding of ATP provides sufficient binding energy to bring the free energy of the enzyme-bound ATP close to that of ADP + Pi so the reaction is reversible
|
|
|
73. With what affinity does F₀F₁ bind ATP?
|
Bind ATP with very high affinity and ADP with much lower affinty
|
|
|
74. What causes the enzyme to release the ATP formed on its surface?
|
The proton gradient causes the enzyme to release the ATP formed
|
|
|
75. What is the binding-change model for ATP synthase?
|
Rotation of ϓ causes sequential ATP synthesis
(120⁰ turn of ϓ = 1 ATP) |
|
|
76. How many subunits does F1 part have?
|
3 nonequivalent subunits
|
|
|
77. How do these three subunits work? At any given moment, one is in the....
Three conformations... |
1. β-ATP conformation (binds ATP tightly)
2. β-ADP conformation (loose-binding) 3. β-empty conformation (very-loose-binding) |
|
|
78. What does the proton-motive force do according to the binding change model?
|
The proton-motive force causes rotation of the central shaft ϓ
|
|
|
79. What does the rotation of ϓ results in?
Three things... |
1. Rotation of ϓ changes the occupancy of each site
2. β ATP site is converted to the β empty and ATP dissociates 3. β ADP site is converted to the β ATP conformation condensing ADP + Pi to form ATP |
|
|
80. What are the requirements for the model?
Two things... |
1. At least two of the three catalytic sites alternate in activity
2. ATP cannot be released from one site unless and until ADP + Pi are bound at the other |
|
|
81. Putting the whole model together, what are the main three things that happen?
|
1. Protons enter Fo
2. This causes rotation of ϓ (turbine) 3. This results in ATP synthesis in F1 (generator) |
|
|
82. What is the P/O ratio?
|
-ATPs/1/2O2 consumed
-Represents the number of ATPs formed per input molecule (NADH or FADH2) |
|
|
83. How much ATP does NADH or FADH2 generate?
|
-It's believed that 4 protons passing through ATP synthase generate 1 ATP
|
|
|
84. What are the P/O ratios for NADH and FADH2?
|
1.(NADH)P/O= 10/4= 2.5 ATP/NADH
2.(FADH2)P/O= 6/4= 1.5 ATP/FADH2 |
|
|
85. If oxidative phosphorylation was 100% efficient how many ATP molecules per glucose can be made?
|
93 ATP/glucose
|
|
|
86. What is the actual yield of oxidative phosphorylation?
|
32 ATP/glucose (or 30 w/ FADH2)
*Under standard conditions only about 30% efficient |
|
|
87. What transport problem is encountered in making ATP and transporting it?
|
In order to make ATP and transport it out of the mitochondrial matrix, specific transport protein must exist
|
|
|
88. Why do we need transport proteins?
Three reasons... |
1. To bring phosphate into the matrix
2. To transport ATP/ADP 3. To transport NADH/NAD+ |
|
|
89. What is used to power the transport of these molecules?
|
The proton gradient!
|
|
|
90. What moves ATP and ADP into and out of the matrix/intermembrane space?
|
Adenine nucleotide translocase
|
|
|
91. Describe the adenine nucleotide translocase.
Three things... |
1. Antiporter
2. Runs off of the electrical gradient 3. Carries ADP/Pi into matrix and newly synthesized ATP into the cytosol |
|
|
92. What moves H+ from the intermembrane space to the matrix?
|
ATP synthase
|
|
|
93. What moves H2PO4- from intermembrane space to matrix?
|
Phosphate translocase
|
|
|
94. Describe the phosphate translocase.
Three points... |
1. Symporter
2. Runs off of the chemical gradient 3. When H+ moves from P side to N side, PO4- goes with it |
|
|
95. Is there any direct way to transport NADH across the inner mitochondrial membrane?
|
NO CHIPPY.
There is no direct way! |
|
|
96. What is one solution to transport NADH across the inner mitochondrial matrix?
|
The malate aspartate shuttle
|
|
|
97. How does the malate shuttle work?
Four steps... |
1. NADH in cytosol is reduced to produce malate
2. Malate crosses inner membrane via the malate α ketoglutarate transporter 3. Malate's reduced and resulting NADH is oxidized by the respiratory chain 4. Oxaloacetate is regenerated in the cytosol |
|
|
98. What is the general gist of the malate shuttle?
|
Take apart on one side and put together on the other
Move electrons out *No waste of energy* |
|
|
99. What is another solution to transport NADH across the inner mitochondrial membrane?
|
Glycerol 3-phosphate shuttle
*This shuttle does not involve membrane transport systems |
|
|
100. What does the glycerol 3-phosphate shuttle do?
|
It delivers the reducing equivalents from NADH to ubiquinone and thus bypasses complex I and produces only 1.5 ATP per pair of electrons
|
|
|
101. What is the difference between a full reduction and a partial reduction of dioxygen?
|
FULL REDUCTION: (4 e-) of dioxygen yields a harmless product (2 water molecules)
PARTIAL REDUCTION: of oxygen makes very toxic and reactive products |
|
|
102. How does the mitochondria get rid of superoxide?
|
Uses superoxide dismutase and glutathione peroxidase which reduce superoxide to water
|
|
|
103. What happens if the proton gradient is dissipated without making ATP?
|
Heat is produced instead
|
|
|
104. What is Brown fat?
|
It's chunky fat!!!!
A special type of adipose tissue whose purpose is to generate heat by dissipating the proton gradient w/o making ATP |
|
|
105. Why is it called brown fat?
|
Because it is rich in mitochondria and cytochromes
SO ITS VERY BEEFY! |
|
|
106. Brown fat contains a certain protein...and this protein is called...?
|
Thermogenin
|
|
|
107. What is thermogenin?
|
A protein that makes a hole in the inner membrane mitochondrial membrane resulting in the dissipation of the gradient
|
|
|
108. What does thermogenin act like?
|
Acts exactly like an uncoupler
|
|
|
109. To whom is brown fat especially important?
|
Babies and hibernating animals
|
|
|
110. What controls the entire process of oxidative phosphorylation?
|
Availability of ADP (substrate availability)
|
|
|
110. Describe the regulation of the complete oxidation of glucose.
Parts 1 and 2 |
1. High ATP (low ADP) reduces:
-glycolysis -pyruvate oxidation -citric acid cycle -oxidative phosphorylation 2. All four pathways are accelerated when the use of ATP and the formation of ADP, AMP, and Pi increase |
|
|
111. Describe the regulation of the complete oxidation of glucose (con't).
Parts 3-5... |
3. Citrate inhibits glycolysis
4. Increased levels of NADH and acetyl-CoA inhibit the oxidation of pyruvate to acetyl-CoA 5. High [NADH]/[NAD+] ratio inhibits the dehydrogenase reactions of the citric acid cycle |
|
|
112. How does photophosphorylation differ from oxidative phosphorylation?
|
Photophosphorylation requires the input of energy in the form of light to create a good e- donor and a good e- acceptor
|
|
|
113. In what ways are photophosphorylation and oxidative phosphorylation similar?
Three things... |
1. Both involve flow of e- through a chain of membrane bound carriers
2. Exergonic e- flow is coupled to endergonic transport of H+ across a proton impermeable membrane 3. Flow of H+ down concentration gradient is couple to synthesis of ATP |
|
|
114. What happens in photophosphorylation?
|
Reverse of oxidative phosphorylation
Pull e- from water and use them to reduce NAD(P) to NAD(P)H for use in making glucose |
|
|
115. What is the ultimate source of carbohydrates and oxygen on planet earth?
|
Plants!
...since both products are derived from photosynthesis |
|
|
116. What are the two stages in which photosynthesis occurs?
|
1. Lights Reactions
2. Carbon Fixation (can occur in light or dark) |
|
|
117. What happens in the light reactions?
|
Make NADPH and ATP (variation of Ox/phos)
|
|
|
118. What happens in carbon fixation?
|
Make carbohydrates by reducing CO2 and using NADPH and ATP (variation on pentose phosphate pathway)
|
|
|
119. Where does photosynthesis occur?
|
In the chloroplast (analogous to mitochondria)
|
|
|
120. What are the parts of the chloroplast?
Four things... |
1. Outer membrane
2. Stroma (site of carbon fixation) 3. Lumen 4. Thylakoid lamellae (site of light reactions) |
|
|
121. What can the thylakoid lamellae be broken into?
Two things |
1. Stromal lamellae (sheets)
2. Grana lamellae (stacks) |
|
|
123. Compare the mitochondria and chloroplast
|
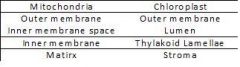
Comparision
|
|
|
124. Describe the big picture of the light reactions.
Two beefy things... |
1. E- move from H2O to PSII, through intermediate chain of carriers, through PSI and finally to NADP+
2. H+ are pumped into the thylakoid lumen, by flow of e- through the carriers linking PSII and PSI,and reenter the stroma through proton channels formed by the Fo |
|
|
125. What is necessary to rip e- from water to generate oxygen?
|
An unusually strong oxidizing agent that is so strong that is usually doesn't exist in normal tissues (stronger than oxygen)
|
|
|
126. What is more electronegative than oxygen?
Can this be used as the strong oxidizing agent? |
F is most electronegative because Adam told us when he was explaining his very boring research.
-It cannot be used though b/c it is too reactive and thus not compatible with life, so Adam's a deush |
|
|
127. If F cannot be used, then how is such a strong oxidizing reagent generated?
|
The strong oxidizing agent is generated with light through photo-oxidation
|
|
|
128. How does photo-oxidation work?
Four steps... |
1. A molecule absorbs a photon of light causing an e- to be promoted to a higher energy state
2. In such a state, the e- is a very powerful reducing agent 3. The e- is easily transferred to another molecule, oxidizing the starting molecule 4. The oxidized molecule can further react by pulling e- from another molecule (i.e. pull e- from water) |
|
|
129. How is photo-oxidation used in photosynthesis?
|
In photosynthesis photopigments (usually chlorophyll) are the molecules that become photo-oxidized
|
|
|
130. What happens to the high energy electron from photo-oxidation in photosynthesis?
|
It is transferred to a specific member of the e- transport chain which will subsequentially use this e- to pump H+ and reduce NADP+ to NADPH
|
|
|
131. What does oxidized chlorophyll do?
|
It pulls e- from water to make oxygen
|
|
|
132. What can happen to a molecule though when it absorbs light?
Three things... |
1. Photo-oxidation
2. Release energy as fluorescence 3. Release energy as heat |
|
|
133. What is photosynthesis designed to ensure?
The most complicated factor... |
The complicated nature of photosynthesis is designed to ensure that only the desired reactions occur
|
|
|
134. What is exciton transfer?
|
When the exciton (quantum of energy) is passes from an excited molecule to another
|
|
|
135. What is chlorophyll?
|
-The most abundant photopigment
-In the thylakoid membrane -Contains chlorophyll a & b -All have long phytol side chain chlorophyll...more like borophyll!!! O'Doya RULES!!! |
|
|
136. What does chlorophyll a absorb?
|
Absorbs violet and red light
|
|
|
137. What does chlorophyll b absorb?
|
Absorbs blue and orange light
|
|
|
138. What are accessory pigments?
|
Secondary light-absorbing pigments
-They scavenge light of other colors -Examples are carotenoids such as β-carotene (red-orange) and lutein (yellow) |
|
|
139. What colors are predominately absorbed by plants?
|
Red and Blue
|
|
|
140. What color has no absorption by plants?
|
Green
|
|
|
141. What are light harvesting complexes?
|
Binding proteins that associate with chlorophyll
(LHC) |
|
|
142. What do light harvesting complexes do?
Four things... |
1. Arrange pigment molecules for optimal activity
2. They act as antennas for collecting light 3. They absorb light energy and transmit it rapidly and efficiently to the reaction center 4. They reduce unproductive side reactions |
|
|
143. What are antenna complexes?
|
Light harvesting complexes
|
|
|
144. What two parts does a photosystem contain?
|
1. The reaction center
2. An electron carrier chain |
|
|
145. What is the reaction center?
|
It converts a high energy electron (formed by the LHC or another photosystem) into chemical energy.
This electron is then transferred to the e- carrier chain |
|
|
146. What important characteristic of carriers does the electron carrier chain have?
|
Carriers that have increasing affinity for electrons
|
|
|
147. What does the fate of an electron depend upon?
|
It depends upon the nature of the photosystem
|
|
|
148. How are photosystems packed in the thylakoid membrane?
|
They are tightly packed w/ several hundred antenna chlorophylls and accessory pigments surrounding a reaction center
|
|
|
149. How do energized electrons get to the reaction center?
|
The antenna complexes pass energized e- to the reaction center with exciton transfer
|
|
|
150. Describe the general conversion of the energy of an absorbed photon.
Five steps |
1. Light excites an antenna molecule raising an e- to a higher energy level
2. Excited antenna molecule passes energy to neighbor exciting it 3. Eventually energy is passed to reaction center exciting it 4. Excited reaction center passes e- to an e- acceptor 5. Electron hole in the reaction center is filled by an e- from an e- donor |
|
|
151. What are the two types of photosystems?
How do they differ? |
Photosystem I and II
They differ in where the (e-)'s come from |
|
|
152. Describe photosystem II.
Three things... |
1. Absorbs light at 680 nm
2. Obtains high energy e- from water 3. Photosynthetic bacteria have similar photosystems |
|
|
153. What is the electron from photosystem II used for?
|
The e- is used to form a proton gradient for ATP production via the cytochrome B6F complex
|
|
|
154. Describe photosystem I
|
1. Absorbs light at 700 nm
2. Obtains high energy e- from PSII |
|
|
155. What happens to the electron from photosystem I?
|
Excited e- is transferred to NADP+ oxidoreductase to make NADPH
|
|
|
156. What couples PSII with PSI?
|
*Mobile electron carrier*
|
|
|
157. What are the three mobile electron carriers in photosynthesis?
|
1. Plastoquinone
2. Plastocyanin 3. Ferrodoxin |
|
|
158. Describe plastoquinone.
Four things... |
1. Two e- carrier
2. Connects PSII and cytochrome B6F 3. Analogous to ubiquinone 4. Write as PQa or Qa |
|
|
159. Describe plastocyanin.
Three things |
1. It's a copper containing protein
2. Soluble one e- carrier 3. Connects cytochrome B6F with PSI |
|
|
160. Describe ferrodoxin.
Four things... |
1. Fe/S center protein
2. One e- carrier 3. Connects PSI with NADP+ oxidoreductase 4. Analogous to cytochrome c |
|
|
161. What is the Z scheme?
|
Shows the pathway of the e- from water to NADP+
-PSI and II are coupled via plastoquinone and plastocyanin *The energy of the e- is boosted twice* |
|
|
162. Which photosystem is a better electron donor?
|
PSI
|
|
|
163. What is the order of electron acceptors starting with the best?
|
1. O2 evolving complex
2. Cyt b6f complex 3. NADP+ |
|
|
164. What are the three protein complexes in photosynthesis?
|
1. Oxygen evolving complex
2. Cytochrome b6f 3. Ferredoxin: NADP+ oxidoreductase |
|
|
165. What does the oxygen evolving complex do?
|
It extracts e- from water for use in replenishing the reaction center in PSII
-O2 is a side product -Protons are released into the lumen |
|
|
166. What does cytochrome b6f complex do?
|
It uses a Q cycles to couple e- flow to proton pumping across the thylakoid membrane into the lumen
|
|
|
167. What does the ferredoxin: NADP+ oxidoreductase do?
|
It uses NADP+ as a final e- acceptor to make NADPH
|
|
|
165. Where does the oxygen evolving complex transfer electrons to?
How many electrons are passed? |
It passes 4 e- one at a time to P680+
|
|
|
165. What is the immediate electron donor to P680+?
|
A tyrosine (Tyr) residue
|
|
|
165. What acts as an electron buffer as the electrons are passed from Tyr?
|
Mn+ complex
|
|
|
165. How does the oxygen evolving complex avoid oxygen radical production?
|
It removes 4 e- at once and there are no toxic or harmful intermediates
|
|
|
166. What is the cytochrome B6F complex analogous to? Why?
|
Complex III
-The reason is b/c it pumps 4 H+ per H2O -It uses the Q cycle so that with 2 e- brought in, 4 H+ can be pumped out |
|
|
168. What do herbicides do to plants?
Besides murder them? |
Many herbicides specifically block photosynthesis
*can order photosynthesis by use of inhibitors |
|
|
169. What is cyclic photophosphorylation?
|
Use photosystems only to make ATP, but not NADPH
-Essentially short circuits the system and drop back again onto the same reaction site |
|
|
170. What is the purpose of oxygen and water molecules in cyclic photophosphorylation?
|
These are molecules to dump e- on or take e- from
|
|
|
176. What does the chloroplast ATP synthase use to make ATP?
|
The transmembrane proton gradient
|
|
|
177. Describe the chloroplast ATP synthase?
|
1. CF1 portion is located on more alkaline (n) side through which H+ flows down its concentration gradient
2. The CFo portion is homologous to mitochondrial Fo |
|
|
178. What is the N side in the chloroplast?
What is the P side? |
The N side is the stroma
The P side is the lumen |
|
|
179. What is the scorecard for photosynthesis?
|
Start with:
2 water 8 photons 2 NADP+ 3 ADP 3 Pi End with: O2, 3 ATP, and 2 NADPH |
|
|
180. How many protons are pumped during photosynthesis?
|
12 Protons
-4 from oxygen evolving complex -8 from b6f complex |
|
|
181. What is the proton to ATP ratio for photosynthesis?
|
4 protons per ATP (same as ox/phos)
|
|
|
182. What are the two ways the high energy electrons are used in photosynthesis?
|
1. By means of a Q cycle, high energy e- are used to pump protons across the thylakoid membrane (PSII)
2. To reduce NADP+ to NADPH (PSI) |
|
|
183. What problem is encountered when comparing PSI and PSII's affinity for electrons?
|
The e- transport chain in PSI has higher affinity for e- than PSII
|
|
|
184. What keeps PSI from directly stealing excited electrons from PSII?
|
Physical interactions between PSII and PSI are carefully regulated
|
|
|
185. How are PSII and PSI physically separated?
Two things... |
1. PSII is in the granal lamellae (appressed membranes)
*stacks* 2. PSI is in the stromal lamellae (nonappressed membranes) *sheets* |
|
|
186. What does the physical separation of PSI and PSII prevent?
|
It prevents PSI from stealing e- from PSII
|
|
|
187. What is the purpose of light harvesting complex II (LHCII)?
Two things... |
1. It controls the ratio of granal to stromal lamellae
2. It's also the "adhesive" that holds appressed lamellae together |
|
|
188. How does LHCII control the ration of granal to stromal lamellae?
|
When the Tyr reside in the hydrophobic domain of LHCII is phosphorylated, we have nonappressed regions (stromal)
When it's not phosphorylated we have appressed regions (granal) |
|
|
189. Why does phosphorylation of the Tyr residue convert appressed regions to nonappressed regions?
|
When the Tyr residue is phosphorylated, LHCII affinity for the neighboring thylakoid membrane is reduced thus converting granal lamellae to stromal lamellae
Stacks->Sheets |
|
|
190. What regulates the phosphorylation of LHCII?
|
Plastoquinol
|
|
|
191. When is LHCII phosphorylated?
When is it not phosphorylated? |
LHCII phosphorylated when protein kinase is activated by high levels of PQH2 (phosphorylate Tyr)
LHCII dephophorylated when protein phosphatase is activated by high PQ levels (dephosphorylate) |
|
|
192. What happens when PSI is as equally active as PSII?
Four things... |
1. Oxidized PQ is plentiful
2. Activity bwt PSI and PSII remain balanced 3. LHCII becomes dephosphorylated 4. The two PS's segregate into granal and stromal lamellae thus preventing direct e- transfer bwt them |
|
|
193. What happens when PSI is less active than PSII?
Five things |
1. Reduced PQH2 is plentiful
2. PSI is upregulated due to this 3. LHCII becomes phosphorylated 4. Granal lamellae turns into stromal lamellae 5. PSI and PSII directly interact |
|
|
194. What is a result of PSI and PSII directly interacting?
Two things... |
1. Electrons from PSII can be directly passed to the PSI e- transport chain
2. LHCII associates w/ PSI to boost its activity |
|
|
195. What does carbon fixation require from the light reactions of photosynthesis?
|
NADPH and ATP
|
|
|
196. What is another name for the process of carbon fixation?
|
The Calvin Cycle
|
|
|
197. What is the Calvin Cycle?
Two things... |
1. A three step cyclic pathway (intermediates serve as catalysts)
2. Three CO2 are fixed for the net synthesis of one molecule of glyceraldehyde 3-phosphate |
|
|
198. What happens in stage one of the Calvin Cycle?
Four points... |
1. Addition of CO2 to ribulose 1,5-bisphosphate
2. Yield (2) 3-phosphoglycerate 3. Catalyzed by RUBISCO 4. KEY REACTION |
|
|
199. What is RUBISCO
Four things... |
1. MOST ABUNDANT enzyme on earth!
2. It's a carboxylase 3. It combines CO2 w/ ribulose 5-Pi to make 2 molecules of 3-phosphoglycerate 4. Central to the proposed mechanism for plant RUBISCO is a carbamoylated Lys side chain w/ a bound Mg2+ ion |

