![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
132 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Define the Primary structure of a Protein.
|
The specific sequence of Amino Acids in a Polypeptide chain
|
|
|
|
Define the Secondary structure of a Protein.
|
The protein structure characterised by folding the peptide chain into an Alpha helix, Beta-pleated sheet or Random Coil.
|
|
|
|
Define the Tertiary structure of a Protein.
|
The overall 3-D shape the chain forms into.
|
|
|
|
Define the Quaternary structure of a Protein.
|
The geometry of several polypeptide chains bound together.
|
|
|
|
How are Amino Acids joined/broken up?
|
J: Condensation reaction B: Hydrolysis reaction |
|
|
|
What are essential Amino Acids?
|
AA that cannot be synthesised in the body, but must come from the diet
|
9 essential AA: Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan and Valine
|
|
|
What are non-essential Amino Acids?
|
AA that are synthesised in our body (even if not eaten)
|
e.g. Alanine, Asparagine, Aspartic Acid, Glutamic Acid etc.
|
|
|
What are conditional Amino Acids?
|
AA that are usually not essential except in times of illness and stress.
|
e.g. Arginine, Cysteine, Glutamine, Tyrosine, Glycine, Ornithine, Proline and Serine
|
|
|
What are Heteromultimeric Proteins? e.g. F1-ATPase
|
Quaternary Structure: The subunits of the multimeric protein's neighbouring strands are different.
|
Multimeric: a protein containing two or more, same or different, polypeptide chains. Monomeric: protein chain is made up of one Chain |
|
|
What are Homomultimeric Proteins? e.g. Homo-oligomeric protein Collagen
|
Quaternary Structure: The subunits of the multimeric protein are all the same
|
Monomeric: protein chain is made up of one Chain |
|
|
Define Saturated Lipid.
|
A chemical compound where Carbon and Hydrogen atoms are bonded by single covalent bonds.
|
|
|
|
Define Unsaturated Lipid.
|
A chemical compound where a double covalent bond (or more) occurs between a carbon and hydrogen atom.
|
|
|
|
Define Cis Lipids.
|
Where the Carbon atom chains are on the same side of the double bond.
|
like Cisters
|
|
|
Define Trans Lipids.
|
Hydrogen atoms are on opposite sides of the Carbon chain's double bond.
|
Trans=opposite
|
|
|
What are the simple and complex Carbohydrates?
|
Simple: Monosaccharides and Disaccharides Complex: Polysaccharides |
M:Glucose, Fructose. Galactose D:Maltose, Lactose, Sucrose P:Starches, fibres, glycogen |
|
|
Structures of Glucose, Fructose and Galactose.
|
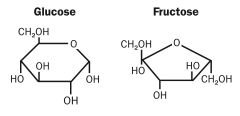
|
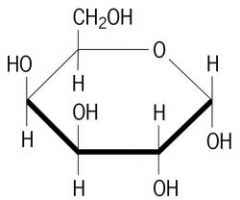
Galactose
|
|
|
What are Sucrose, Maltose and Lactose made up of?
|
S: Glucose+Fructose M: Glucose+Glucose L= Galactose+Glucose |
Note: Homopolysaccharides are composed of one type of Sugar monomer. |
|
|
Where are the glycosidic bonds in Starch?
|
a(1+4)
|
Where a=alpha symbol, 1 and 4 are carbon positions
|
|
|
Where are the glycosidic bonds in Glycogen and describe its structure?
|
a(1+4), glycosidic links main chain with fewer a(1+6) branches
|
|
|
|
Name a structural Polysaccharide and describe its structure.
|
Cellulose: B(1+4) glycosidic main chain links. H-bonds are also present between main branches |
B=Beta
|
|
|
Give examples of proteins in the body and their functions.
|
1. Enzymes: Catalysis 2. Antibodies: Defence 3.Haemoglobin: Transportation 4. Collagen: Support 5. Actin/Myosin: Motion 6.Hormones: Reg./Communic. 7. Ferritin: Storage (Fe) |
|
|
|
Give examples of Lipids
|
1. Adipose Tissue 2. Phospholipids, vitamins 3. Hormones, Prostaglandins 4. Myelin Sheath, Subcutaneous fat |
1. Energy Storage 2. Provide Building, Vit. ADEK 3. Communication 4. Thermal/electrical insulation and protection |
|
|
What protein polymers make up the cytoskeleton? (Also flagella/cilia of cells)
|
1. Tubulin: Microtubule protein component 2. Actin: Microfilament protein component 3. Lamin: Intermediate Filament protein component |
In contrast to actin filaments and microtubules, the intermediate filaments are not directly involved in cell movements. Instead, they appear to play basically a structural role by providing mechanical strength to cells and tissues.
|
|
|
What is the function of Microtubules?
|
Acts as 'Scaffolding', Transports vesicles and molecules via molecular motors (Dynein and Kinesin) |
|
|
|
What are MAPs?
|
Microtubule Associated Proteins: any protein that interacts with the microtubules of the cellular skeleton.
|
|
|
|
What is the function of MAPs?
|
One of its domains binds to tubulin polymers or unpolymerized tubulin. This speeds up polymerization, facilitates assembly and stabilizes the microtubules |
The other end projects out and will bind to vesicles or granules, IF or other MT.
|
|
|
What is the function of Actin Filaments?
|
1. Helps the cells change shape 2. Moves organelles and larger cell component (e.g. choromosomes) |
|
|
|
What is a 'Pathway'? 'Intermediates'?
|
1. A set of consecutive reactions 2. Components of the Pathway |
Products never occur in isolation, the product f one becomes a substrate in another.
|
|
|
Define Anabolic.
|
Pathways that generate complex molecules from smaller substrates. (Consume energy) |
e.g. Glycogenesis, anything with -genesis
|
|
|
Define Catabolic.
|
Pathways that breakdown complex molecules into smaller products. (Tend to release intrinsic chemical energy)
|
e.g. Glycolysis, anything with -lysis
|
|
|
Define Amphibolic.
|
A biochemical pathway that involves both catabolism and anabolism.
|
e.g. Krebs Cycle
|
|
|
Gibbs Free Energy is used when the reaction occurs spontaneously. What does it tell us?
|
How much energy will be released in the process
|
Note: It is independent of the pathway/reaction mechanism
|
|
|
What does H, T, and S stand for in the Gibb's Free energy reaction?
|
Delta H: Enthalpy Change T: Temperature Delta S: Entropy Change |
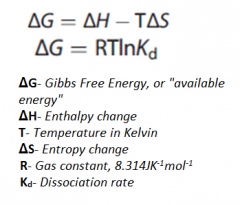
|
|
|
Free energy changes are additive, energy yield reactions can...
|
Be coupled to unfavourable/Endothermic ones to drive the reaction.
|
|
|
|
Outline ATP Coupling.
|
ATP gives off energy, coupling it with other reactions can allow endothermic reactions to happen spontaneously.
|
|
|
|
ATP->ADP+Pi. How much energy is associated with this reaction?
|
-30.5Kj/Mol
|
|
|
|
ATP->AMP+PPi. How much energy is associated with this reaction?
|
-45.6Kj/Mol
|
|
|
|
What is ATP?
|
A universal energy carrier and phosphoryl carrier.
|
|
|
|
What is Phosphocreatine?
|
An energy store in muscle (Creatine Phosphate->Creatine +Phosphate;-43.1Kj/Mol) |
a.k.a Creatine Phosphate Transfers Pi to ADP->ATP via Creatine Kinase |
|
|
What is the function of Kinases?
|
Phosphorylates molecules and sometimes the reverse. (Though this is the primary function of Phosphatases) |
|
|
|
What is NAD+? Function? Structure?
|
1. Nicotinamide Adenine Dinucleotide 2. Accepts H+ and 2e- (NADH+H+) 3. Nicotinamide ring synth. from Vitamin B3 |
|
|
|
What is NAD+? Function? Structure?
|
1. Flavin Adenine Dinucleotide 2. Accepts 2H+ and 2e- (2H) 3. Riboflavin (Vitamin B2) |
|
|
|
Describe the structure of ATP and how it relates to its function.
|
S: Adenine ring attached to Ribose Sugar and a tail of 3 phosphate groups. F: 2 Phospho-anhydride bonds release energy when hydrolysed. |
|
|
|
What are the 5 functions of ATP?
|
1. Energy/Phosphate carrier for many reactions 2. Intracellular Signalling 3. Adenine Nucleotide Synthesis 4.ATPase (Active Transport) |
1. Phospho-anhydride bonds 2. cAMP? 3. For DNA/RNA Synthesis 4. Certain enzyme requires a constant supply of it. |
|
|
What are some sources of ATP?
|
Substrate-level Phosphorylation Oxidative Phosphorylation (of ADP)
|
|
|
|
Why is NADH2 sometimes used instead of NADH+H+?
|
It simplifies the expression, H+ is still produced and dissolved into the solution of the cell.
|
|
|
|
What is NAD+? Function? Structure?
|
1. Nicotinamide Adenine Dinucleotide Phosphate 2. Redox Partner 3. Like NAD, but has a Phosphate group at C2 |
|
|
|
What are Acetyl-CoA's 4 functions?
|
1. A 2 Carbon Carrier 2. Generates ATP directly when oxidised 3. A substrate for many synthetic pathways 4. Most cellular catabolic pathways eventually lead to Acetyl CoA |
|
|
|
Describe Acetyl-CoA's Structure?
|
1. Acetyl group linked to Coenzyme A (Pantothenic Acid) 2. Functional Group: Thiol group, which forms thioester bonds |
Acetyl group (CH3COO-) Thiol group (-SH) Sometimes called CoA-SH |
|
|
Which enzyme is used in Redox Reactions?
|
Dehydrogenase enzymes
|
e.g. Malate to Oxaloacetate in Krebs Cycle
|
|
|
What is Ligation? Enzyme?
|
Adding smaller things together to make something bigger. Ligase |
|
|
|
What is Isomerisation? Enzyme?
|
Rearrangement of existing atoms within substrate molecules. Isomerase |
Citrate to Isocitrate
|
|
|
Which enzymes do Group Transfers?
|
Transferase Kinases (specifically phosphate groups) |
Puts the functional group of one molecule to another.
|
|
|
What do Lyases do?
|
Enzymes which catalyse the joining of specified molecules or groups by a double bond
|
e.g. Aldolase
|
|
|
What are the 5 ways pathways are regulated?
|
1. Synthesis vs Breakdown of enzyme 2. [S] vs [P] 3. Substrate Availability 4. Allosteric & Covalent Modification 5. Adenylate control and energy charge |
1. (More: Faster) 2. Le Chatelier's Princiciple 3. B-Oxidation vs Fatty Acid Synthesis 4/5. Regulation of Catalytic Activity |
|
|
What is Rate Limiting Step? What does it determine?
|
The slowest step in a metabolic pathway/chemical reaction series It determines the overall rate of other reactions in the pathway |
Note: usually irreversible |
|
|
What are the energy requirements for Men and Women in KCal and KJ?
|
M: 2500KCal/10500KJ F: 2000/8400KJ |
|
|
|
What does Km tell you? (mM)
|
The affinity of a transporter to what's being transported (Whether or not the rate of formation of product will be affected by the availability of substrate).
|
The lower the Km the more affinity, the more saturated the enzyme is.
|
|
|
Where is GLUT 1 found? Km? Special Properties?
|
1. Most cells 2. 1-2; High Affinity 3. High capacity, Basal Uptake |
|
|
|
Where is GLUT 2 found? Km? Special Properties?
|
1. Liver, B-Pancreatic, Small Intestine 2. 15; Low Affinity 3. High Capacity, Glucose Sensor in Beta-Cells, Glucose/Fructose carrier in small intestines |
1. Hepatocytes, insulin producing cells & Enterocytes 2. High or low affinity? |
|
|
Where is GLUT 3 found? Km? Special Properties?
|
1. Neuron, Placenta, Testes 2. 1; High Affinity 3. High Capacity, Basal Uptake |
|
|
|
Where is GLUT 4 found? Km? Special Properties?
|
1. Fat, Skeletal and Cardiac Muscle 2. 5 3. Insulin Activated |
|
|
|
Where is GLUT 5 found? Km? Special Properties?
|
1. Mucosal Surface of Small Intestines and Sperm 2. N/A 3. Primarily a Fructose Carrier |
|
|
|
Outline the Glucose Transport mechanism of Na+-glucose symporter
|
Sodium Pump: Pumps Na+ out and Glucose in. Secondary Active Transport: Na+ electrochemical gradient is used to take Glucose in. |
|
|
|
GLUT 4 is insulin responsive... GLUT 2 has a high Km... |
1. The more you exercise the more you have. 2. So a higher glucose concentration is required for transportation |
|
|
|
Where does Glycolysis mainly occur?
|
In cells with few or no mitochondria. When O2 supply is insufficient. |
|
|
|
Glycolysis: What is the first step? Requires... |
Hexokinase phosphorylates Glucose into Glucose 6-Phosphate Uses ATP |
|
|
|
How is the first step of Glycolysis regulated?
|
It is inhibited by its own product (G-6P) Allosteric Regulation: G-6P non-competitive inhibition |
Negative and Positive Modulators exist.
|
|
|
Inhibitors and Activators are...
|
Known as Allosteric Modulators
|
|
|
|
In Liver: The enzyme is...
|
A different isoform (Glucokinase), the enzyme has a low affinity for Glucose (High Km). Not Allosteric |
|
|
|
Glycolysis Step 2: What does Phosphoglucose Isomerase do?
|
It converts Glucose 6-Phosphate into Fructose 6-Phosphate
|
|
|
|
Glycolysis Step 3: What does Phospho-fructo Kinase do? (PFK) Requires...
|
1. Fructose 6-Phosphate into Fructose-1,6-Phosphate (Most important regulatory step) 2. ATP |
|
|
|
How is Phospho-fructo Kinase regulated?
|
1. Adenylate Control 2. pH Control |
Adenylate: Allosteric Regulation, ATP and ADP switch off AMP blocks allosteric site, positive modulators |
|
|
Any differences in the liver?
|
Down-regulated by: Citrate Up-regulated by: F-2,6-bisphosphate |
|
|
|
Glycolysis Step 4: Fructose-1,6-bisphosphate is turned into...
|
1. Dihydroxyacetone Phosphate (DHAP) 2. Glyceraldehyde 3-Phosphate (GAP) Enzyme: Aldolase |
Intermediate: Aldol, enzyme named after it GAP a.k.a. Triose Phosphate |
|
|
DHAP is useless as it is so...
|
Triose Phosphate Isomerase turns it into Glyceraldehyde 3-Phosphate (GAP)
|
GAP a.k.a. Triose Phosphate
|
|
|
Glycolysis Step 5: Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH)... Requires... |
Turns GAP into 1,3-Bisphosphoglycerate NAD+ |
|
|
|
Glycolysis Step 6: Phosphoglycerate Kinase turns... What else happens? |
1,3-Bisphosphoglycerate into 3-Phosphoglycerate (3-PG) Substrate-level Phosphorylation |
|
|
|
Glycolysis Step 7: Phosphoglycerate Mutase turns...
|
3-Phosphoglycerate (3-PG) into 2-Phosphoglycerate (2-PG)
|
Mutase: Group Transfer, within same molecule
|
|
|
Glycolysis Step 8: Enolase/Phosphopyruvate Hydratase (a Lyase) turns... Gives off... |
2-Phosphoglycerate (2-PG) into Phosphoenol Pyruvate (PEP) Water Molecule |
|
|
|
Glycolysis Step 9: Pyruvate Kinase turns... What also happens?
|
1. Phosphoenol Pyruvate (PEP) into Pyruvate. 2. Substrate Level Phosphorylation |
|
|
|
3 Regulatory Points...
|
Down regulated by: 1. ATP allosteric mechanism 2. Alanine Feed-Forward Mechanism 3. Fructose-1,6-bisphosphate |
1. Glucose Conservation 2. Synthesised by Pyruvate 3. Switches on Pyruvate Kinase |
|
|
How Pyruvate Kinase differ in specific tissues?
|
1. Liver 2. Gluconeogenic Tissues (Muscle and Brain) Conserves PEP by being inhibited by ATP and AA |
1. L-Form2. M-Form
|
|
|
Outline Lactate Formation.
|
Pyruvate gets reduced via Lactate Dehydrogenase NADH is converted back into NAD+ allowing Glycolysis to continue. |
Glycolysis occurs in the cytoplasm
|
|
|
What is the fate of Lactate?
|
It's moved to the liver and is converted into glucose via Gluconeogenesis Or oxidised to pyruvate for TCA, by well oxygenated muscle, heart and brain cells |
Converts in to Pyruvate, then Glucose reversing the
|
|
|
The Link Reaction occurs between TCA and Glycolysis. What happens in it?
|
1. Pyruvate get decarboxylated and dehydrogenated to Acetyl CoA 2. By Pyruvate Dehydrogenase
|
|
|
|
What are the 4 cofactors of Pyruvate Dehydrogenase?
|
1. Thiamine Pyrophosphate 2. Lipoic Acid 3. CoA 4. NAD+ |
Committed Irreversible Step: Reaction cannot stop after this until the product is formed. |
|
|
How is the Link Reaction regulated?
|
1. Under Adenylate Allosteric regulation 2. Up regulated by: NADH+H+, Acetyl CoA and Ca++ |
|
|
|
Where does the Tricarboxylic Acid Cycle (TCA) occur? (Consists of 8 reactions)
|
Mitochondrial Matrix
|
|
|
|
TCA Step 1: Acetyl CoA combines with... Enzyme? What is released? |
1. Oxaloacetate to form Citric Acid/Citrate (6C) 2. Citryl Synthase 3. CoA released |
Citrate is an allosteric effector: Inhibits PFK-1 Activates Acetyl-CoA Carboxylase |
|
|
TCA Step 2: Citrate (6C) is converted into...
|
Isocitrate (6C) By Aconitase |
|
|
|
TCA Step 3: Isocitrate (6C) is dehydrogenated and decarboxylated (Oxidative Decarboxylation) into...
|
Alpha-Ketoglutarate (5C) By Isocitrate Dehydrogenase |
Inhibited by high ATP and NADH+H+ levels, which raises Citrate to accumulate C02 |
|
|
TCA Step 4: Alpha-Ketoglutarate (5C) goes through Oxidative Decarboxylation to become...
|
1. Succinyl-CoA (4C) By Alpha-Ketoglutarate Dehydrogenase 2. Gives off: NADH+H+, CO2
|
|
|
|
TCA Step 5: Succinyl-CoA (4C) is converted into...
|
Succinate (4C) By Succinyl CoA Synthetase/ Succinate Thiokinase Produced: Thioester Bond's energy conserved via GTP, CoA freed |
GTP formation from GDP and Pi; Substrate Level Phosphorylation
|
|
|
TCA Step 6: Succinate (4C) is oxidised into...
|
Fumarate (4C) By Succinate Dehydrogenase FAD is reduced to FADH2 |
A trans-dicarboxylic acid By a flavoproteiin containing the prosthetic group FAD |
|
|
TCA Step 7: Fumarase adds water to Fumarate (4C)...
|
At the trans double bond, forming Malate (4C)
|
Alpha-hydroxyl acid L-Malate
|
|
|
TCA Step 8: Malate 4C is oxidised into...
|
Oxaloacetate (4C) By Malate Dehydrogenase Produces: NADH+H+ |
May become Citrate again by repeating cycle
|
|
|
What is the functions of the TCA cycle?
|
1. Generates reducing equivalents for Oxidative Phosphorylation 2. Intermediates of the TCA are Raw Materials for anabolic pathways |
NADH+H+ AND FADH2 Note: TCA is an Amphibolic Pathway |
|
|
Which 3 steps are allosteric and irreversible? (Rate-limiting)
|
1. Step 1: Citrate Synthase 2. Step 3: Isocitrate Dehydrogenase 3. Step 4: Alpha-Ketoglutarate Dehydrogenase |
|
|
|
Intracellular Ca++ is elevated when energy demanding processes are active, therefore...
|
Calcium ions allosterically activate the 3 enzymes so they operate more rapidly (Positive Modulator)
|
e.g. Muscle Contraction, Cell Division and Exocytosis of Neurotransmitters
|
|
|
Negative Modulators/Allosteric Inhibitors of TCA?
|
NADH+H+ and ATP Their abundance reflects high cellular energy level |
|
|
|
What 4 factors regulate the TCA cycle?
|
1. Substrate Availability 2. Supply of NAD+ & FAD 3. Acetyl CoA availability 4. Increased rate of Oxidative Phosphorylation |
|
|
|
List uses of TCA intermediates as raw materials:
|
1. Oxaloacetate for Gluconeogenesis 2. Citrate for A-CoA for fatty acid and cholesterol synthesis 3. Alpha-ketoglutarate and Oxaloacetate in AA synthesis 4. Porphyrin/haem synthesis via Succinyl CoA |
|
|
|
Bonus: Define Anaplerotic Reactions.
|
Pathways and Reactions which replenish pathway molecules e.g. Pyruvate Carboxylation replenishes Oxaloacetate |
|
|
|
Why is Substrate-Level Phosphorylation important?
|
No need for oxygen, vital for rapidly contracting skeletal muscle.
|
e.g. Step 5 of TCA, 7 and 10 of Glycolysis and Phosphocreatine Hydrolysis via Creatine Kinase in Muscle cells |
|
|
What is the function of ATP-ADP Translocase?
|
Saps the electrical gradient by 25% to: 1. Transport ATP to the cytoplasm 2. ADP into the Mitochondrial Matrix |
|
|
|
One turn of the TCA cycle generates... How many ATP per Acetyl CoA? Per Glucose? |
1. 1 FADH2, 3 NADH+H+ 2. 10 ATP is generated per Acetyl CoA 3. 30-32 moles of ATP per Glucose |
FADH2= 1.5 ATP NADH+H+= 2.5 ATP and 1 GTP molecule which is worth 1 ATP Anaerobic is just a net of 2 ATP |
|
|
Why is the theoretical yield of ATP never met?
|
1. Some energy is lost as heat 2. Not every step is 100% efficient. 3. Some Protons leak out of memebrane 4. ATP is required for shuttles |
|
|
|
Why is it called Oxidative Phosphorylation? |
1. Electron Pairs are transferred between ETC complexes. 2. ADP is phosphorylated; forming ATP |
|
|
|
How is a H+ chemical gradient set up? |
The electron movement in an electronegative direction, releases energy. |
|
|
|
How is ATP made in oxidative phosphorylation. |
H+ flows down conc. gradient back into mitochondrial matrix from intermembrane space via ATP synthase. Provides energy for ATP. |
|
|
|
ETC consists of... |
4 protein structures embedded in the IMM |
Each structure is numbered in order of increasing electron affinity and redox potential. |
|
|
How is a H+ chemical gradient set up? |
The electron movement in an electronegative direction, releases energy. |
|
|
|
How is ATP made in oxidative phosphorylation. |
H+ flows down conc. gradient back into mitochondrial matrix from intermembrane space via ATP synthase. Provides energy for ATP. |
|
|
|
ETC consists of... |
4 protein structures embedded in the IMM |
Each structure is numbered in order of increasing electron affinity and redox potential. |
|
|
NADH+H+ transfers 2H+(+2e-) to complex I. FADH2... |
Transfers two electrons and protons. |
|
|
|
How is a H+ chemical gradient set up? |
The electron movement in an electronegative direction, releases energy. |
|
|
|
How is ATP made in oxidative phosphorylation. |
H+ flows down conc. gradient back into mitochondrial matrix from intermembrane space via ATP synthase. Provides energy for ATP. |
|
|
|
ETC consists of... |
4 protein structures embedded in the IMM |
Each structure is numbered in order of increasing electron affinity and redox potential. |
|
|
NADH+H+ transfers 2H+(+2e-) to complex I. FADH2... |
Transfers two electrons and protons. |
|
|
|
Why does FADH2 generate less ATP? |
Proton pumping occurs at III and IV unlike NADH+H+ where it occurs at I, III and IV |
FAD and NAD+ are restored |
|
|
Complex III receives electrons and protons from... |
Complex I or Complex II via coenzyme Q |
|
|
|
Complex III's electrons and protons reach Complex IV... |
Via Cytochrome C |
Electron transfer is highly exergonic. The final electron pair acceptor is O2. |
|
|
Which Complexes pump protons from the mitochondrial matrix into intermembrane space? |
I, III and IV, sets up proton gradient for Chemiosmosis |
|
|
|
What is Complex V? |
ATP Synthase Catalyses Phosphoanhydride bond formation of ADP and Pi |
|
|
|
ATP is coupled with proton gradient discharge... |
Chemiosmotic coupling |
|
|
|
Define Uncoupling protein. |
An inner mitochondrial matrix that can dissipate the proton gradient before it can be used to provide energy for oxidative phosphorylation. |
e.g. H+ ions discharge back into mitochondrial matrix through a normal proton pore; so no ATP |
|
|
What are the advantages of Uncoupling? |
If heat is required restores body temperature |
Usually in hairless newborn mammals. |
|
|
Newborn babies have specialised heat-generating cells, what are they? |
Brown fat cells, they have a large number of uncoupled mitochondria for heat production. |
|
|
|
Free radicals are molecules containing an unpaired electron. Function? |
Oxidative Damage: enter undesirable redox reactions Adding, inflammation, diabetes complication. |
Causes: Radiation, Smoking Cure: antioxidants mop up, catalase enzymes |
|
|
Iron switches between ferrous and ferric states? What are these? |
Ferric: Fe3+ Ferrous: Fe2+ |
|
|
|
What regulates Oxidative Phosphorylation? |
Determined by ATP demand Controlled by ADP, until ATP increases |
|

