![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
176 Cards in this Set
- Front
- Back
|
What is a conformation? |
It is when molecules have one single bond between them so they can cange direction without braking covalent bonds. |
|
|
What is a configuration? |
It is when molecules have doble bonds or a chiral center. Bonds need to be broken to change direction (the configuration) |
|
|
It is two different doble bonds, what are they and what differenciates them? |
Cis: paralell Trans: Not paralell |
|
|
How many aminoacids do have a chiral centers? |
All, but Glycine which have a Hydrogen as a R- group. |
|
|
What is stereoisomers |
Two molecules with the same chemical formula and bonds, but different configuration |
|
|
Life depends on the electronegativity of? |
Oxigen |
|
|
Is the attraction between molecules less or more if the electrogravity is high? |
More attraction = more electrons/electro negativity |
|
|
WHat is an atom? |
A atom is f.exampe hydrogen. "H" |
|
|
What is a covalent bond? |
A strong interaction |
|
|
What is steriechemistry? |
It is the study of the 3D structures of molucules |
|
|
What is a chiral atom? |
-Usually a carbon (which is the chiral centre) that is connected to four different substituent groups. - Not superimposable on its mirror image |
|
|
What is a chiral molecule? |
It is a molecule with one ore more chiral centers, and are not able to become it's mirror image without braking any bonds. (Superimposable on it's mirros image) |
|
|
What ia a molecule? |
It is when two or more atoms are connected |
|
|
What are the chemistry in living organisms organized around? |
Carbon: around 50% of dry weight of the cell |
|
|
What kind of bonds can form between two carbon-atoms? |
Single, double and tripple bonds (3 bonds are not usual) |
|
|
How big chain can carbons form with single bonds? |
Five carbon can atach to eachother with only single bonds |
|
|
Where does the four bonds form on the carbon? |
They project form the four apices of tetrahedron |
|
|
Is it free rotation between the single bonds between carbons? |
Yes, but the rotation can be restricted if highly charged groups are attached to the carbons |
|
|
what kind of function has double bonds? |
They are shorter and more rigid -> more rigid/less movement between bounded atoms |
|
|
What is the major chemical element in molecule-forming? |
Carbon |
|
|
Most biomolecules is regarded as derivates from hydro-carbons. What goups are mentionated in the lectures? |
Alcohol - hydroxyl groups Amines - amino groups Aldehydes + Ketons - carbonyl group Carboxylic acids - carbonyl groups. |
|
|
Why has distance between nuclei and electrons an importans in the strength of chemical bounds? |
The closer a electron are the nuclei, stronger are the chemical bond, because the electrons in the outer shell losing some of the appeal to the ones before them. |
|
|
WHat does it mean that a biomolecule are polyfunctional? |
A molecule has two or more types of functional grops with their own chemical reactions and characteristics. |
|
|
What does the chemical personality / chemical reactions depends on? |
Their functional groups and their disposition in the three dimentional space |
|
|
The Acetyl-coenzyme A is a carrier of acetyl-groups in enzymatic reactions, how many functional groups does it have? Are the groups in protonated or nonprotonated forms? |
8 functional grops that are in protonaded or unprotonaded forms depending on the pH. |
|
|
Proteins are dynamic molecules, what does their function depend on? |
THe interaction with other molecules, which can give subtible/small or stricing/big changes in the proteins conformation. This is very importaint for physiological processes. |
|
|
Can proteins functions involve reversible binding between binding site and ligand? |
Yes |
|
|
What is a ligand, and where does it bind to the protein? |
A ligand are a molecule which interact with the protein by binding at spesific points called "binding sites" |
|
|
What happens in catalyse reactions? |
It is when a molecule, which in this case are called a SUBSTRATE, binds to an enzyme at the active site/ catalytic site, and are chemically transformed |
|
|
What is the main function of hemoglobin? |
Hemoglobin is a protein that are responsible for oxygen transport inside the bloodstream |
|
|
WHat is the main function of myoglobin? |
It is a protein that store oxigen inside the muscles, and realise it incase of stress. |
|
|
What two atom-binding has the strongest single bond? |
Oxygen - hydrogen (461kJ/mol) Hydrogen - Hydrogen (435kJ/mol) |
|
|
Can oxygen bind reversable to amino acid-chains in proteins? |
No, so in the case of hemoglobin and their work with transporting oxygen form lungs to tissue do they need a heme-group that consist of a complex organic ring of protoporphyrin and an Iron in the middle. |
|
|
How many coordination bonds does an Iron atom inside the hemegroup have, and how are they placed? |
6 bonds:' Four that are plane and are bound to the porphyrin ring system. Two that stands out in the other directions: one that binds it to the protein and one that reversable form a covalent bond to the oxygen. |
|
|
What is stereochemistry? |
Study of the 3D strucure of molecules |
|
|
What is the difference between R-state and T-state? |
R-state (realaxed): higher affinity to oxygen(lung) T-state (tense): lower affinity to the oxygen(tissue) |
|
|
Does the hemglobin only binds to oxygen in the R-state? |
No, but it is a bigger change to bind to the oxygen: higher affinity |
|
|
What stabilize the R-state of haemoglobin? |
The oxygen binding |
|
|
What is the key-words for the T-state of hemoglobin? |
- More interactions: more stabile - Low affinity for O2(oxygen) - Tissue |
|
|
What is the key-words for R-state of hemoglobin? |
- Fewer interactions: more flexible - High affinity for O2 - Lungs |
|
|
What triggers the T-state to transform into R-state? |
Oxygen binding triggers the conformational change from T-state into R-state: change the positions of the individual subunits, which make the space between the iron and the His7 narrower and increase the affinity for oxygen (between the beta-subunits) |
|
|
what is enantiomers? |
It is isomeres that are a mirror image of eachother. |
|
|
What is Diastereomers? |
Isomers(same primary structure/different secondary structure) that are not mirror image of eachother |
|
|
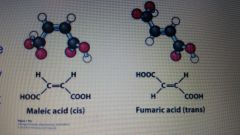
What is the isomer of Maleic acid(cis)? |
Fumaric Acid (trans) |
|
|
What is geometric isomers/ cis-trans isomers? |
It is molecules with exact same binding, but have different configuration (double bond) |
|
|
what are the active sie in enzymes lined up of? |
Amino acid residues |
|
|
What desides if you have high or low electronegativity? |
The amount of electrons compared to protons: - higher ammount of electrons = high electro negativity (negative charged) - Lower amount of electrons = low electro negativity (positive charged) |
|
|
What is atimic number(Z)? |
It is the number of protns in the atom nucleus |
|
|
What is neutral nuclear charge? |
Same number of protons(+) and electrons(-) |
|
|
What is effective nuclear charge? |
It is the charge experienced form the outest electron-orbital |
|
|
What 6 types of biochemichal reactions do we find? |
1. Oxidation-reduction 2. Group transfer 3. Condensation/hydrolisis 4. Bond cleavage/formation 5. Double bond cleavage/formation 6. Rearrangement |
|
|
What is oxidation-reduction? |
When a molecule oxidise, an other are redused. Oxidation: electron loss, energy produced Reduction: electron gain. |
|
|
How does living organism obtain energy? |
Oxidation of fat and carbohydrates: metabolic fuels |
|
|
Can carbons attach a hydrogen bond? |
NEVER |
|
|
What is endergonic reactions? |
Reactions that requires energy (positive charge with an higher amount of these reactions inside th cell) |
|
|
What is exergonic reactions? |
Reactions that realise free-energy (negative charge with a higher amountof these reactions in the cell)) |
|
|
IS the main goal for the cells exergonic or endegonic processes in catabolism? |
Exergonic- negative charged as the sum of free energy |
|
|
What is an electrophile? |
electro-deficient atom : more nucleus than electrons |
|
|
What is an nucleophile? |
nucleus-defisient atom: more electrons than protons |
|
|
When O2 (oxigen) has an high affinity for electrons, is this exergonic or endergonic?????? |
Exergonic?????????????? |
|
|
What can redistribution (omfordeling) of electrons produce? |
Internal rearangements, isomerization and eliminations (oxdation/reduction, cis-trans rearangement, transpotition of double bonds) |
|
|
What type of group-transfer is speacially importaint in cells? Required for activation of molecules for reactions that otherwise would be highly unfavorable. |
Phosphoryl transfer: phosphate-groups are moved (often ATP: allenine three phosphate) |
|
|
What does the strength of chemical bonds depend on? |
- Electronegativity: electron attraction - Distance between nuclei and electrons - Number of electrons - Nuclear charge |
|
|
What is condensation/hydrolisis? |
Hydrolisis is when the reaction are using water to brake the bond, while the reverable reaction are realising water when making a bond: condensation |
|
|
What is a heterotropic clevage? |
It is when two atoms cleavage (brake their bond) and one gets both of the electrons. Most common type of cleavage. : Two carbons: carboncation (+) and carbonanion (-) One hydorgen and one carbon: the hydogen becomes a proton (+) or an hydrogen ion |
|
|
What is an homolytic cleavage? |
It is when the bond between two atoms brake and they gets equal number of electrons each: one electron each. Not common. Carbon radicales. |
|
|
What is rearrangement of molecules? |
Different configuration of conformation |
|
|
What are macromolecules polymers of? |
Monomers |
|
|
What kind of week interactions do we find in aqueous systems? |
1. Hydrogen bond 2. Ionic interactions 3. Hydrophobic interactions 4. Van der Waals interactions |
|
|
What is hydrogen bonds? |
It is a weak interaction between oxygen and hydrogen: dipole are created when the hydogen are slithely positive and oxygen slithely negative. No real shared elecron- they only like to be close. |
|
|
What is ionic interaction |
Interaction between one positive and one negative charged molecules: Ions: |
|
|
What is Van der Waals interactions |
Two uncharged atoms that are near eachother, so the electron can be attrachted to the other atoms proton, so a little small dipole are created: transet electron dipole. This is the weakest interaction. |
|
|
What makes water liquid or crystalized depending on the temperature? |
The hydrogen bonds between water molecules. Four hydogenbond in ice. In liquid are the watermolecules always moving so hydogenbonds are braked and formed rapitly. |
|
|
Can hydrogenbond form with carbon? |
No |
|
|
Why are salt desolved in water? |
They are disolved by ionisation: Water molecules is dipole (HO2 - the hydrogen is positive and the oxygen is negative) - The salt is also dipole (Na+Cl- positive sodium and negative clorine) = Clorine and hydrogen interact/ oxygen and sodium interact) |
|
|
Hydrophobic interactions |
Week interactions: |
|
|
What is polar and non-polar? |
Polar: can be positive charged(interact with water) Non-polar: |
|
|
What does it mean that lipids can be amphipatic? |
Lipids that have some polar parts (hydrophilic) and some non-polar parts (hydrophobic) |
|
|
What are creating all biological membranes? |
Phosphorlipids |
|
|
What is the Van der Waalsradius? |
How near the atoms can come near eachother before they are pushed away. |
|
|
What are theweek interactions controlling? |
The chemical reactions: substrate-enzyme/ligand-protein |
|
|
What is osmosis? |
It is when the water moves through the semipermimale membrane due to the consentration of solids: Isotonic, hypotonic, hypertonic |
|
|
What is an isotonic, hypotonic and hypertonic solution? |
Isotonic: no net water movemet Hypertonic: solid-consentrationis very high outside -> water moves out of cell: cell shrink Hypotonic: Solid-consentration is high inside-> water moves inside cell: can explode |
|
|
Theeffect on solutes in osmolarity depends on mass or number of dissolved particles? |
The number! |
|
|
osmotic pressure |
the force/pressure needed to resist water movement |
|
|
What kind of equilibrium constant does pure water have? |
55,5 M |
|
|
What is an acidic enviorment? |
High consentration of protons and low PH (less than7) |
|
|
Basic enviorment |
Few protons: pH higher than pH 7 |
|
|
Are pH realeted to eactions between molecules |
Yes: molecules can be active in different Ph'es |
|
|
Is acetyl-soenzyme A realated in metabolism? How many functional groups does it have? |
8 functiaonal group, and realated on metabolism |
|
|
What is an Oleic acid? |
It is an fatty acid that occures naturally in the metabolism: It is an example of doble bond/cleavage formation: where the oleic asid are condensed: carbons binds doble covalentely to each other and realises water. |
|
|
Rearrangement |
When the molecule has te same structure, but change the organisation by braking bonds. |
|
|
When a solution is released into water and an H+ is loose, what is it? |
An acid |
|
|
What does this sentence mean? "Weak acid and weak bases are in equilibrium with its conjugate pair. |
?????????????? |
|
|
What is dessosiation constant(Ka)? |
Describes the tendensy for an acid (proton donor) to lose its proton. |
|
|
What kind of acids/bases are complitely ionizide |
Strong acids and strong bases |
|
|
How is pH and Ka realated? |
Low Ka (the proton donors tendenzy to give away its protons is low = HIGH pKa -> pH: Base |
|
|
wich is the proton donor and proton acceptor of acids and bases? |
base= acceptor Acid = donor |
|
|
What does the henderson - Hasselback |
- Calculate pKa given the pH and the molar ration of proton donor and acceptor
• Calculate pH given the pKa and the molar ration of proton donor and acceptor • Calculate the molar ration of proton donor and acceptor given the pKa and the pH |
|
|
What kind of buffersystem are in vivo? |
Phosphate Histidine (bloodplasma) Bicarbonate (contol PH-range) |
|
|
What does solubility of polar molecules depends on? |
H-bonds acceptors and donors |
|
|
How does two cyteine becomes an cytine |
They oxidize by realisin two prtonan and two electrons |
|
|
WHich two amino acids do not form protein but are importaint in methabolism? |
Citrulline and ornithine |
|
|
What does it mean when in ionizable amino-side chine can be titrated? |
It is when it has three pK's or buffering regions, two base and one acid, or two acids and one base. (pI will be calculated by the mean of the two bases or the two acids. |
|
|
How is the formation of peptide bonds? |
Condensation between two amino acids: realise water to bind ny peptide bon. this is reversable: hydrolises. |
|
|
What is the differen tbetween the oligopeptide and the polypeptide? |
Oligopeptide: when a few aminoacids are bonded to eachother Polypeptide and proteins: when a lot of aminoacids are connected. |
|
|
Is polypeptide bons or covalents bonds strongest? |
peptidebond |
|
|
In an aminoacid-chain, which end do we start with, and which two ends exist? |
only one free alfa-carboxyl(end) and one alfa-amino group(start)
|
|
|
Is the partial doulbe bond flexible? |
No, they can not rotate, because a small dipole are created when the electrons prefer the oxygen than the other atoms |
|
|
Why is the C-N bond shorter than others? |
Because it is a partial doble bond |
|
|
WHat is proteins combined of? |
It is polypeptides (which is alfa-amino acids covalently bond(polypeptide bonds) + cofactor, coenzymes, prostetic groups or other modifications. |
|
|
Can all proteins function alone? |
No, sometmes they need an other molecue to function |
|
|
Monomeric protein? |
It is a protein of only one polypeptide chain |
|
|
Multimeric protein |
It is a protein consisting of two or more non-covalently bounded polypeptides. |
|
|
WHat two classifications can be used of proteins? |
1. Multimeric/monomeric 2. Simple/conjugated |
|
|
What is the difference between simple and conjugated proteins? |
Simple: ONLY amino acids Conjugated: It is a protein-complex: permanated assosiated to other components (prostetic group) |
|
|
cofactors |
Additional chemical component to some proteins: inorganic components or coenzymes (complex organic or metaloorganic molecule) |
|
|
coenzymes |
Complex organic or metaloorganic molecule |
|
|
Prostetic group |
Coenzyme or metal ion that is tightly bounded, maybe even covalently attached (heme in myoglobin) |
|
|
Native protein |
The active conformation/structur/position of a protein |
|
|
Stabile |
the proteins tendensy to maintain the native conformation |
|
|
What does the protein structures depends on ? |
The primary structure (amino acide sequence) and the weak interactions: hydrogen bonds, hydrophoic interactions, ionic interactions and Van der Waals. |
|
|
What make the protein function? |
primary structure (sequence) and the tetriary structure (conformation) |
|
|
What is an secondary structure? |
It is the local spartial arrangement of the polypeptide with weak interactions: alfa-helix, beta-sheets, Beta-turns |
|
|
Beta-turns |
Where a regular pattern is not found, the secondary structure is sometimes referred to as undefined or as a random coil.
Allow changes in directionConnect two consecutive helixesor two segments of a sheetFour amino acids• High frequency of Gly (aa3) and Pro (aa2) |
|
|
Beta- sheet |
Peptide bonds create a pleated sheet-like structure
Held together by hydrogen bonds between groups in different strands Side chains protrude from the sheet alternating in up and down direction Parallel (weak H bonds) or antiparallel (strong H bonds) orientation of two chains within a sheet are possible High frequency of small aa (Gly, Ala) |
|
|
Alfa-helix |
- stabilized by hydrogen bonds by nearby residues. - a primay sequence around a axis: aminoacids are arrange around it. - R groups protrude outward. - Each helical turn 3.6 aa - Stabilized by HB - Each turn 3-4 HB that confers stability. - opposite charged are near eachother |
|
|
Which amino acids will we not find in alfa-helix? |
Glysine (small) and Proline (rigid) |
|
|
what special about Bulky/big R-groups in alfa-helix structure? |
They will make an distance between eachother and make structural/functional differences: destabilizes the structure. |
|
|
WHich interactions between the amino acid side chains in the alfa-helix stabilize the structure? |
Hydogen bonds Ionic bridges |
|
|
WHich interactions between the amino acid side chains in the alfa-helix destabilize the structure?
|
-Lysin -Bulk -glycine and Proline - terminal changed amino acide residues |
|
|
Tertiary-structure |
Tertiary structure refers to the overall spatial arrangement of all atoms in a proteinStabilized by numerous weak interactions between amino acid side chains.• Largely hydrophobic and polar interactions• Can be stabilised by disulfide bondsInteracting amino acids are not necessarily next to each other in the primary sequence. Two major classes• Fibrous (long strands or sheets) and globular (spherical or globular shape)
|
|
|
What are the difference between fibrous and globular classification inside the tetranary structure? |
Fibrous ones are realatad to strength: form hair, collagen, silk, bones
Globular ones are realated to regulation, methabolism, oxygen transport and antibodies |
|
|
What is supersecondary structure? |
- It is a MOTIF (recogizable folding pattern) in the tertrinary structure (NOT IN THE SECONDARY STRUCTURE). - This can be elements of the secondary structures connecting and forming an fold and representing an little part of the protein or may comprise the whole protein - a protein is formed by different motif folded together: some with spesific functions |
|
|
quaternary structure |
Assembly of individual polypeptides into a larger functional cluster
Different functions in one complex Sequenced reactions Regulation realated - 4-5 polypeptides working together |
|
|
What is intrisically disorders? |
It is a part of the protein which has no spesific 3D strutrure (no alpha-helix, beta-sheets and beta-turns): it can help the protein to adapt envorments easylier, : present in some regulatory proteins. Contain protein segments that lack definable structureComposed of amino acids whose higher concentration forces lessdefined structure• Lys, Arg, Glu, and ProDisordered regions can conform to many different proteins,facilitating interaction with numerous different partner proteins |
|
|
Intrinsically disordered proteins |
Contain protein segments that lack definable structureComposed of amino acids whose higher concentration forces lessdefined structure• Lys, Arg, Glu, and ProDisordered regions can conform to many different proteins,facilitating interaction with numerous different partner proteins
|
|
|
Fibrous protein: collagen, collagenchain, collagen fibril |
Collagen is an important constituent ofconnective tissue: tendons, cartilage, bones,cornea of the eye
Each collagen chain is a long Gly- and Prorich left-handed helix Three collagen chains intertwine into a righthanded superhelical triple helix: STRENGTH Many triple-helices assemble into a collagenfibril |
|
|
What happens when the body lack ascorbate(vitamin lack)? |
Proline can not form into 4-hydroxyproline -> problem in the collagen of the body -> illness |
|
|
Globular proteins |
Compact structureStructure-function diversityHydrophobic residues insideStructural domainsStructural homologies
- regulatory functions |
|
|
Denaturation and renaturation |
-Denaturation: the moment when the native conformation is lost
-Renaturation: when the native conformation is changed back (due to pH or temprature) |
|
|
What kind of protein-folding can occure? |
- Spontaneous folding (not that common) - Chaperones: a family of proteins involved in protein folding to prevent missfolding(use ATP)- two major families: Hsp 70 and chaperonins |
|
|
Amyloidosis and spongiform encephalopathy |
Missfoldings: 1)Amyloidosis: Transormation of normal proteins with a lot of betasheets, this is realated to parkinsons and alzeimers 2)Spongiform Ensephalopathy: Caused by prions in neurons, if they change, they change the function: realated t o mad cow disease |
|
|
Proteostasis |
-The continual maintenance ofthe active set of cellular proteins requiredunder a given set of conditions(spesific number ofproteins are needed for a cell to function) • Protein synthesis and folding• Refolding of proteins• Sequestration and degradation ofproteins that are irreversiblyunfolde |
|
|
What kind of interactions occure between ligand and protein(binding sites)? |
Week interacirons (non-covalent) |
|
|
How do you calculate the assosiation constant, and what does it stand for? |
The assosiation constant (Ka) stands for the affinity between ligand and protein: Ka= PL(consentratin of protein bound to ligand=product)/P(consentration of protein) times L(consentration of ligands) |
|
|
What does the fraction of bound sites depends on? |
Free ligand consentration and Kd (dissosiatin constant=ligand consentration nessesary for 50%saturatin)) |
|
|
What does it mean that the ligand and protein has indused fit? |
That the ligand or the protein can have an conformational change to fit eachother better, have an higher affinity |
|
|
Why are theheme-group attached to a protein? |
Because without the protein, it would attached to the high consentration of carbonmonocyte and not the low consentration of oxygen: the distal histedine E7 make the room less comfortable for the carbonmonocyte: less affinyty for the carbonmonoxyte (CO) |
|
|
WHy does we say that the haemoglobin is tetramere nin their quatrinary structure? |
It has two alfa and two beta subunits: four polypeptidechains. |
|
|
How does theaffinity change due to pH? |
Low pH - Low affiity |
|
|
Hemoglomin in the T-state realises oxygen into the tissue, the cells resive the oxygen and give Co2: carbondioxide, what happens here? |
CO2 is an gas and can not travel trough the bloodstream alone, so it need to transform: |
|
|
haemoglobin has cooperative |
Positive homotropic allosteric regulationOne binding to the oxygygen results in even more bining of same type of ligand (oxygen) : affinity increase in case of the conformation |
|
|
Does carbondioxide solve in bloodplasma? |
No, its apolar |
|
|
Allosteric protein |
– Binding of a ligand to one site affects the binding properties of a different site, on the same protein– Binding of a ligand induces a conformational change– Can be positive or negative– Homotropic• Normal ligand of the protein is the allosteric regulator– Heterotropic• Different ligand affects binding of the normal ligan
|
|
|
2,3 biphosphoglycerate (BPG) is a heterotropic regulator
|
• Binds to and stabilises the T state (makes it possible to realise the oxygen in tissue)
• Reduces O2affinity • Foetal Hb (alfa2gamma2) has low affinity for BPG - does not compete about the oxygen binding-site: binds to an positive chared aminoacid in the beta subunit |
|
|
What differ the fetal heamoglobin from the adult one? |
The fetal blood has less oxygen consentration, so it need to have a higher affinity to oxygen, and a lower affinity to the BPG(heterotropic regulater): gamma sub-units instead of beta-subuntis |
|
|
What makes it possible for us to adapt to high alterns? (mountains) |
Low consentration of oxygen-> less oxygen in lungs -> less oxygen to realise in tissue (normal realise is 38%) To stabilize this does the BPG-consentration increase ->easyer to realise the oxygen in the tissue(37%) |
|
|
Sickle cell animea |
One single mutation of one singe amino acid, change the hemamoglobin -> fibous transformation: insoulable -> change the shape of the red bloodcells (harder to get malaria) |
|
|
Biocatalysis |
Enzymes that speed up reactions or slow them down: physiological condition (3D structure) |
|
|
What makes the enzyme active? |
Spesific conformations |
|
|
Isonzymes |
It is two enzymes with the same function, but are codifyed by different genes(different enzymes) |
|
|
Ribozymes |
RNA with catalytic function(The exeption enzymes that are not made out of amino acids) |
|
|
What 6 different group are the enzymes classifed under due to their function? |
1. Oxidoreductases: trranfer of electrons (hydrideinon or Hatoms) 2. Transferases: group transfer reactions 3. Hydrolases: hydrolysis reactions(transer of r-group to water) 4.Lysases: cleavage of c-c, o-c,c-n, or others by elimination , leaving double bond or rings, or addition of groups to double bonds. 5. Isomerases: transfer of groupswithing the molecule to yield isomeric forms 6. Ligases: formation of covalent bonds condensatin reactions coupled to cleavege of ATP or simular cofactors |
|
|
NAD/NADP |
Derived from niacin(vitamin B) Interchange one hydride ion (:H-, two electrons)They can dissociate from the enzyme after the reactionNAD+participates as oxidant in catabolismNADPH participates as reducing agent in anabolisChanges in [NADH] can be monitored by UVspectrophotometryMeasure the change of absorbance at 340 nmVery useful signal when studying the kinetics of NAD-dependent dehydrogenases
|
|
|
What is the main cofactors and coenzymes? |
- NAD/NADP:(oxidise/reduction: measure abrorbanse 340nm) - FMN/FAD(tightly bound to protein:electron interchange reactions) - ATP(energy domor->exergoic reactions) |
|
|
Enzyme-substrat binding |
The substrate are bound and transformed at the active site(a spesific potition on the enzyme) |
|
|
What does the enzymes affect? |
Only the reaction rate, NEVER the reaction equilibrium. They affect the reaction rate(speed) by reduce the activation energy needed to reach the transition state->incrase of velosity. |
|
|
What does the enzyme velosity depends on? |
The number of substrate |
|
|
What types of catalytic mechanisms do we find? |
Acid-base catalysis: give and take protons
Covalent catalysis: change reaction paths• A transient covalent bond between the enzymeand the substrate Metal ion catalysis: use redox cofactors• A metal ion bound to the enzyme interacts withsubstrate to facilitate binding Electrostatic catalysis: preferential interactions with TS |
|
|
To obtain an product most effectly, what is the "key" for the bindingsite and substrate? |
The bindingsite/enzyme must be completely cmplimentary to the transition state: makes the substrate to work until its perfectly complimentary to the binding-site. |
|
|
Exergonic/endergonic reaction |
Exergonic: negative free energy: realise energy Endergonic: positive free energy: consume energy |
|
|
Activation energy |
Enzyme-substrate: the energy needed to transform substrate into product: reach the transition state (when the reaction reach its highest energy point) |
|
|
Is the molecule more or less stabile when they have much energy? |
Less stabile |
|
|
What can enzymatic reactions e affected by? |
• enzyme• substrate•effectors• temperature
|
|
|
what kind of "TWO_SUBSTRATE"reactions is there? |
1)Enzyme reaction involving tertinary structure: - Randomised: both substrate bindsat ones and are formed into product at the same time - Ordered: One substrate binds before its able to bind with the second, and then transform both at the same time 2) enzyme reactions with no tertinary complex: fist transform one subrtrate into product, then binding to the second and transfoming te second one into product. |

