![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
107 Cards in this Set
- Front
- Back
|
By what steps does a DNA sequence become a protein? |
DNA sequence is transcribed to an mRNA sequence which is spliced and then transcribed using tRNA into an amino acid chain which folds into a protein |
|
|
What can proteins be involved in? |
Structural, movement, immunity, endocrine systems, transport, biological catalysts |
|
|
What is the amino acid structure? How do you make it acidic/ basic? |
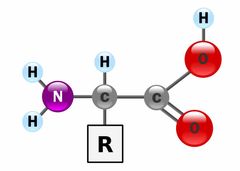
Back (Definition) |
|
|
What is the primary structure of a protein? |
Chain of amino acids joined by a peptide bond via condensation reactions |
|
|
What is the secondary structure of a protein? |
Hydrogen bonds formed between peptide groups to form an alpha helix or beta pleated sheet |
|
|
What is the tertiary structure of a protein? |
Amino acids forming a specific shape protein by forming more hydrogen bonds, ionic bonds, covalent bonds, disulphides bridges, electrostatic attractions |
|
|
What is the quaternary structures of protein? Give examples and state how many strands they have. |
Interaction between different protein chains to form one large protein. Collagen= 3 Haemoglobin= 4 |
|
|
How do enzymes work? |
Enzymes= biological catalysts that decrease the activation energy by bringing substrates together, stabilising the transition state, transfer chemical groups This causes the the rate of reaction to increase |
|
|
What are the types of enzyme-substrate binding? Describe them. |
Isosteric- rate increases as substrate increases until enzymes become saturated Allosteric- substrates and/or effectors induce change in the enzyme that increases activity- substrate binds causing a favourable change in the active site so triggers more substrates to join |
|
|
What examples do we have of allosteric regulation? |
Glutamine synthase converts glutamate to glutamine Amino acid and nucleotide biosynthesis |
|
|
What type of enzyme inhibitors are there? |
Competitive- fit into active site of enzyme Non-competitive- binds to allosteric site to change active site shape Uncompetitive- binds to enzyme-substrate complex Allosteric- usually non/uncompetitive |
|
|
What factors affect enzyme activity? |
Concentration, pH, temperature, co-factors, post transitional modifications |
|
|
What examples do we have of post transitional modifications to affect enzyme activity? |
Phosphorylation- changes shape of enzyme active site to increase or decrease rate Proteolytic activation- cleave the peptide bonds to form fully functional form of enzymes eg- proteinase= enzyme which cleave proteins, serine protease= serine at active site, metalloproteinases= metal ions at active site Co-enzymes + cofactors- ions or compels organic molecules help enzyme reactions take place |
|
|
What is an isoenzyme? |
Different enzymes that catalyse the same reaction- derived from different genes in different tissues |
|
|
What is a multi-enzyme complex? |
Several enzymes/ co-enzymes in one functional unit to perform a multi-step transformation and increase the rate by decreasing the side reactions |
|
|
What are the types of proteins? |
Globular Fibrous Membrane |
|
|
What are the features of globular protein? |
Compact structures, usually soluble, most proteins are globular, interact with smaller molecules |
|
|
What are the features of fibrous proteins? |
Multiple strands bonded together, insoluble structures |
|
|
What is the structure of keratin? |
Single strands held together but disulphides bridges, 4 protofibriils form a microfibrils, microfibrils come together to form a macrofibril |
|
|
What is the structure of silk? |
Fibroin is beta pleated sheet with side chains of flying, alanine or serine |
|
|
What is the structure of collagen? |
Polypeptide of helixes, 3 twisted around each other to form a super helix |
|
|
What are the defects of collagen and what can this cause? |
Pro-collagen N-proteinase which results in malformed fibrils= fragile and hyper elastic skin |
|
|
What is prions disease? |
Prions disease- infectious proteins, cause host normal prions to switch to a confirmation that readily aggregates- aggregation into fibres in the brain which can cause neural cell death |
|
|
What examples do we have of prions disease in animals? |
Scrapie BSE Mad cow disease |
|
|
What is a monosaccharide? |
Simple carbon sugar |
|
|
What is classical von gierkes disease? |
Glucose-6-phosphatase deficiency- liver can’t convert glucose-6-phosphate to glucose= bloody hypoglycaemia= enlarged liver due to excessive glycogen storages |
|
|
What is pompes disease? |
Deficiency in acid alpha glucosidase- leads to hypoglycaemia and abnormal enlargement of the liver Normally degrades maltose- without it no degradation= lysosomal accumulation of glycogen |
|
|
What is cori’s disease? |
Deficiency of debranching enzyme= shorter and more frequent glycogen branches= hypoglycaemia and enlarged liver |
|
|
What is type 1 diabetes? |
Selectively destroying islet cells In dogs= immune-mediated destruction of beta cells In cats= deposition of amylin in pancreas |
|
|
What is type 2 diabetes? |
Normal/ high insulin but no response to glucose loading= insulin resistance |
|
|
What is a disaccharide? |
2 monosaccharides joined together by a glycoside bond via condensation reaction |
|
|
What is a polysaccharide? |
Several monosaccharides joined together by a glycosidic bond formed in a condensation reaction |
|
|
What is chitin a repeat of? Where is it found? |
N-acetyl-beta-glucosamine = insect skin |
|
|
What are n-linked glycoproteins? |
Occurs at ASN amino acid- nitrogen atom is added in ER and modified in golgi N between ASN and N-acetyl glucosamine |
|
|
What are o-linked proteins? |
Occurs between serine/ threonine and n-acetyl galactosamine- binds to an oxygen atom.
The other carbohydrates can be added Happens in golgi |
|
|
Where does glycogen breakdown occur? |
Liver and muscles |
|
|
What does muscles use for energy in glycolysis and why? |
Glucose-6-phosphate as they don’t have the glucose-6-phosphatase enzyme to break it down |
|
|
How is glycogen broken down? |
Glycolysis 1) glycogen phosphorylase releases glucose-1-phosphate from glycogen 2) this is converted to glucose- 6- phosphate 3) this is then broken down into glucose (not in muscles) |
|
|
How is glycogen formed? |
Glycogenesis 1) glucose-6-phosphate is converted back to glucose-1-phosphate by phosphoglucomutase 2) it’s then converted into UDP-glucose 3) glycogenin is a primer enzyme and is primed by x8 UDP-glucose 4) glycogen is formed from primed glycogenin using glycogen synthase and UDP-glucose |
|
|
What are the steps of glycolysis? |
Converting glucose to pyruvate by 10 enzymatic reactions
1) hexokinase converts glucose to glucose-6-phosphate (in the liver, already like this in muscle) 2) converted to fructose ring 3) preparation phase- uses 2 ATP 4) Fructose-6-carbon ring is split into 2 3 carbon molecules. 5) pay-off phase forms 4 glucose |
|
|
What happens in anaerobic glycolysis? |
Pyruvate is converted to lactate which regenerates the NAD+ for glycolysis to continue |
|
|
What 2 chemicals cause toxicity on aerobic respiration and how? |
Sodium fluoroacetate- binds to CoA, provides a metabolite for aconitase
Cyanide- prevents transfer of electrons to oxygen= disrupts ETC= can’’t produce ATP so rapidly affects the heart and CNS |
|
|
What is the difference between plasma and serum blood? |
Plasma= contains plasma proteins Serum= clotted sos no plasma proteins |
|
|
What can you add to plasma blood to do different biological tests? |
Heparin= biochemical tests but not glucose Fluoride= test blood glucose EDTA= haematology |
|
|
Why do you have to use fluoride tubes for measuring blood glucose? |
Because the fluoride stops the red blood cells metabolising so prevents false decreased readings of blood glucose |
|
|
What is an activated carrier and give examples? |
They temporarily store the energy released by biochemical reactions Eg: ADP, NAD, NADP+, FAD CoA |
|
|
How many molecules of glucose are: A) made B) used C) net yield In glycolysis |
A)4 B)2 C)2 made |
|
|
How are carbohydrates metabolised in non-ruminants? |
Polysaccharides are broken down to monosaccharides which are transported across the intestinal epithelial cells. These are then are converted to ATP by glycolysis, Krebs cycle and oxidative phosphorylation |
|
|
How are carbohydrates metabolised in ruminants? |
Fermentation converts carbohydrates to VFAs (acetate, propionate, butyrate), lactate, CO2, hydrogen and methane Propionate is the used in gluconeogenisis to form glucose to then be used in respiration |
|
|
What are the key steps of gluconeogenesis in ruminants? |
Propionate is converted to propionyl CoA This is converted to MethylMalonyl CoA by ATP-> AMP This is converted to Succinyl CoA This is converted to oxalactate which is converted to pyruvate and then glucose |
|
|
What situations would gluconeogenisis be needed in animals? |
To turn propionate from ruminants fermentation to glucose Starvation= depletion of glycogen storage= amino acids and fatty acids converted to glucose Intense exercise= lactate build up= converted to glucose after exercise |
|
|
What is the basic structure of a fatty acid? |
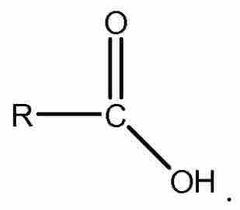
Back (Definition) |
|
|
What is the basic structure of a triglyceride? |
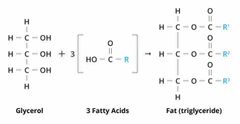
Back (Definition) |
|
|
What’s the basic structure of a phospholipid? |
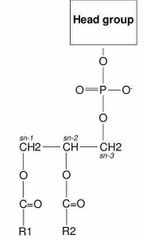
Back (Definition) |
|
|
What is the basic structure of a glycolipid? |
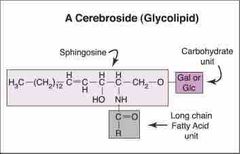
Back (Definition) |
|
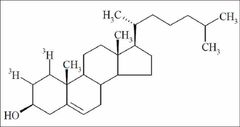
What is this the structure of? |
Cholestrol |
|
|
What is the basic structure of a glycolipid? |
Back (Definition) |
|
|
What is this the structure of? |
Cholestrol |
|
|
What’s the difference between a saturated and unsaturated fatty acid? |
Unsaturated has a C=C, saturated does not |
|
|
What is the role of cholesterol? |
It’s a precursor for lipid soluble hormones (corticoids, androgens, estrogens)
This allows them to pass through the bilayer |
|
|
What is arachidonic acid? |
Unsaturated fatty acid that can bee present in phospholipids. Acts as a signalling molecule and inflammatory mediator- if animal can’t synthesis it must be in diet (cats) |
|
|
What is the role of aspirin? |
NSAID that inhibits the synthesis of prostaglandins (lipids that act at a tissue damage site.) |
|
|
What species is aspirin toxic to? |
Cats |
|
|
List roles of lipids |
Cell membranes, fuel, heat insulation, signalling molecules, vitamins (fat soluble- DEAK), nervous system |
|
|
How are lipids digested and absorbed in non-ruminants? |
1) bile salts emulsify dietary fat in the small intestine forming micelles 2) lipase break down triglycerides into fatty acids and glycerol 3) fats are reassembled and added to chylomicrons 4) these are secreted into the lymph system and are transported releasing fatty acids and glycerol a to cells or to the liver |
|
|
What happens in hydrolysis of a triglyceride? |
It breaks down into glycerol and 3 fatty acids |
|
|
What is beta-oxidation? |
A catabolic process where fatty acid molecules are broken down in the mitochondria. This generates several molecules of acetyl-CoA. Beta carbon on the fatty acid undergoes oxidation to a carbonyl group |
|
|
What happens to the last carbon on an odd numbered fatty acid chain in beta-oxidation? |
Leaves a propionyl-CoA at the end which is converted to Succinyl-CoA which can then be converted to glucose |
|
|
What happens in ketosis? |
High levels of ketone bodies causes metabolic acidosis. Gluconeogenisis in the liver converts the ketone bodies to glucose but uses the intermediate from the citric acid cycle meaning they can’t be used in the cycle so acetyl-CoA can’t enter the cycle meaning ketone bodies are used as the fuel |
|
|
What is the nitrogen cycle? |
A process where nitrogen is converted between various chemical forms |
|
|
What is symbiotic nitrogen fixation? |
Occurs in plants with nitrogen fixing bacteria in their tissues, usually rhizobium The soil and legumes exchange nitrogen for other essentials eg carbs |
|
|
How is nitrogen in the soil converted to ammonia? |
Nitrogen in the soil in the form of nitrate. Nitrate is reduced into nitrite by nitrate reductase enzyme Nitrite is then reduced into ammonia by nitrite reductase |
|
|
How is ammonia converted into amino acids? |
NH3 in plants is present as NH4+ (Amino acid) dehydrogenase converts the ammonia to an amino acid |
|
|
How is glutamate produced from ammonia? |
Glutamate dehydrogenase converts ammonia to glutamate with the use of NADPH—> NADP+ |
|
|
What amino acids can be produced from glutamate directly? |
Glutamine, proline, arginine Using ATP |
|
|
What amino acids can be made from glutamate added to oxaloacetate (from the TCA) |
Aspartate which can be converted to asparagine, methionige, threonine, lysine |
|
|
Name the essential amino acids |
Arginine, histidine, isoleucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine |
|
|
What is the biogenic amino acid of aspartate? |
Alanine= component of coenzyme A |
|
|
What is the biogenic amino acid of threonine? |
Amino-propanol= component of vitamin B12 |
|
|
What is the biogenic amino acid of cysteine? |
Cysteamine= component of coenzyme A |
|
|
What is the biogenic amino acid of serine? |
Ethanolamine= lipid component |
|
|
How are amino acids broken down? |
Glucogenic amino acids are broken down to TCA cycle intermediates- which can be converted to glucose by gluconeogenesis Ketogenic amino acids are broken down to acetyl-CoA or acetoacetate which can be converted to ketone bodies |
|
|
What is transamination? |
A chemical reaction between an amino acid and ketoacid where they swap and the ketoacid becomes the amino acid and vice versa |
|
|
What is deamination? |
Removing of nitrogen from the ketoacid/ amino acid to produce urea |
|
|
What is tryptophan used for? |
It’s a precursor for serotonin- neurotransmitter A sleep aid and anti depressant |
|
|
What is leucine used for? |
Additive flavour enhance. Slows muscle deflation by increasing muscle protein |
|
|
Who should avoid cysteine and why? |
Diabetics as it interacts with insulin |
|
|
What amino acids can be used as neurotransmitters and what type? |
Glutamate- important main excitatory neurotransmitter Glycine- inhibitory neurotransmitter Aspartate- rare excitatory neurotransmitter |
|
|
What is the biogenic amino acid of glutamine? |
GABA Inhibitory transmitter |
|
|
What is the biogenic amino acids of tyrosine? |
Dopamine= inhibitory neurotransmitter Nor-adrenaline= hormone and neurotransmitter Adrenaline= hormone and neurotransmitter |
|
|
What is the biogenic amino acid of tryptophan? |
Serotonin= neurotransmitter and local mediator Melatonin= neurotransmitter and hormone |
|
|
What is the biogenic amino acid of histidine? |
Histamine= neurotransmitter and local mediator |
|
|
What is the biogenic amino acid of arginine? |
Agmatine= neuromodulator |
|
|
Where is urea produced? |
The liver |
|
|
How do birds and reptiles excrete nitrogen? |
They excrete it as uric acid (uricotelic) This is produced by the synthesis of purine nucleotides. Degradation of these produce uric acid (Complex pathway that requires energy) |
|
|
What can cause kidney and bladder stones in Dalmatians? |
They have a deficiency in uric acid breakdown |
|
|
What do ruminants do to digest proteins from their diet? |
Most are hydrolysed to amino acids by rumen microbes. These are used for the microbes own protein synthesis or are degraded to ammonia which is transferred to the liver to be converted to urea and excreted. Some escape this and are digested in the small intestine where the amino acids can be absorbed |
|
|
What amino acids are essential to a ruminant and why? |
None because dietary non-protein nitrogen (including urea) can be incorporated into proteins and the microbes in the rumen can synthesis all 20 amino acids |
|
|
What is the product of transamination and deamination? |
NH4+ |
|
|
How does nitrogen enter the urea cycle? |
NH4+ from the liber is converted to carbonyl phosphate which van enter the urea cycle |
|
|
How are ammonium ions transported in the blood? |
They’re converted to alanine or glutamine |
|
|
How is urea secreted? (Include ruminants) |
Kidneys and in saliva and sweat In ruminants it’s excreted into the gastrointestinal system where it is used for amino acids biosynthesis |
|
|
What can cause a high blood urea nitrogen? |
Increased protein catabolism Increased protein digestion Decreased glomerular filtrate rate |
|
|
What can cause lower blood urea nitrogen? |
Decreased protein intake Increased protein synthesis Increased excretion rate Decreased urea production |
|
|
How is nitrogen excreted in aquatic animals? |
They directly excrete ammonium- this is called ammonotelic |
|
|
Where are ammonium ions generated in a fish? |
The girls via deamination |
|
|
Which fish can produce urea as well as ammonia? |
Sharks and other cartilaginous fish |

