![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
Thermodynamics relating tl biochemistry |
Thermodynamics is the study of the energetics of chrmical reactions (two forms of energy in chemistry: kinetic, and potential energy) |
|
|
Kinetic energy |
Movements of molecules |
|
|
Potential energy |
Energy stored in chemical bonds |
|
|
What is the most important potential energy storage molecule in all cells? |
ATP, which stores energy in the ester bonds between it's phosphate groups. |
|
|
The first law of thermodynamics, also known as the law of conservation of energy states that |
Energy of the universe is constant, energy can't be created nor destroy. Implying that when the energy of a system decreases, the energy of the rest of the universe must increase and vice versa. |
|
|
The second law of thermodynamics states that the disorder, or entropy, of the universe tends to increase. Another way to state this is by saying that |
Spontaneous reaction tends to increase the disorder of the universe// Causing a change in entropy (/\S delta S) |
|
|
Free energy formula (Gibbs free energy) |
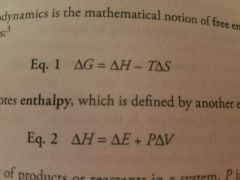
T= temperature, H= enthalpy |

