![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
83 Cards in this Set
- Front
- Back
|
5 Characteristics of Living Organisms
|
1. Complex and dynamic - Biomolecules and biochemical reactions
2. Organized and self-sustaining - Hierarchial order (atoms->organism) and a constant influx of energy and matter. Also has enzyme regulated metabolic pathways. 3. Cellular - cell membranes regulate entrance and exit of crap 4. Information-based - DNA 5. Mutations -> Adaptation and evolution |
|
|
Organic Functional Groups
|
alkene
alcohol thiol aldehyde keton ester amine amide carboxylic acid |
|
|
Small and Large Biomolecules
|
Amino Acids -> Peptides, Polypeptides and Proteins
Monosaccharides -> Carbs and polyglycans Fatty Acids (lipids) Nucleotides -> Nucleic Acids |
|
|
4 Classes of Large Biomolecules
|
Carbohydrates
Lipids Nucleic Acids Proteins |
|
|
Structure and bonding of Amino Acids
|
Amino group, Carboxylic Acid group, Side Chain
Peptide/Amide bonds - has double-bond character that impacts overall structure |
|
|
Individual amino acids inside proteins are called
|
residues
|
|
|
Polypeptides vs peptides vs proteins
|
Peptides and proteins are TYPES of polypeptides
Peptides have 50 or fewer amino acids Proteins have more than 50 amino acids |
|
|
Structure and function of sugars/Carbz
|
contain alcohol groups + carbonylz (aldehydres in aldoses and ketones in ketoses)
Functions - energy, structure, communication |
|
|
Structure and function of fatty acids
|
Long hydrocarbon chains with a carboxylic acid group.
They are a component of lipids, so fat soluble but not water soluble Triglycerols store energy Phophoglycerides (like phospholipids) function in membrane |
|
|
Structure and function of Nucleotides
|
Five-carbon sugar, 1 or more phosphate groups, and purine/pyrimidine base (2/1 ring nitrogenous base)
Super important in biosynthetic and energy-generating reactions GA = Pure, 2 rings UTC = Pyrimidines, 1 ring CG = 3 bonds AT = 2 bonds |
|
|
ATP is a type of...
|
Nucleotide??
|
|
|
Structure of nucleic acids
|
nucleotides with phosphodiester linkages
|
|
|
Big organic functional groups in biomolecules
|
Phosphoester - OPOR
Phosphodiester - ROPOR Phosphoanhydride - OPOPO Anhydride - OCOCO |
|
|
Autopoiesis
|
Autonomy, self-organization and self-maintenance
|
|
|
Most Common Biochemical Reactions
|
Nucleophilic Substitution (hydrolysis)
Addition Elimination (hydration) Isomerization Redox |
|
|
Anabolism vs catabolism
|
ANA builds biomolecules and the cat claws them apart (biosynth vs degradation)
ANA uses energy and the cat stores it up Uses electrons vs capturing Uses oxidation of NADH, FADH2 and NADPH vs reduction of NAD, FAD and NADP+ |
|
|
Self-assembly
|
biomolecules in supramolecular structures are able to assemble because of the STERIC INFORMATION they contain
|
|
|
Macromolecular crowding
|
cells are densely crowded. Macromolecular crowding is a significant factor in a wide variety of cellular processes
|
|
|
Words that are related to signal transduction lol
|
signals
Neurotransmitters hormones cytokines ligands |
|
|
Structure of Prokaryotic cells
|
cell wall and plasma membrane. Has nucleoid
|
|
|
Vesicular organelles
|
examples include acid hydrolase-rich lysosome, plant vacuoles, granules, and melanosomes.
|
|
|
Peroxisomes
|
generate and break down peroxides - glyoxsomes in plants
|
|
|
Plastids
|
plants, algae and some protists - sites of manufacture and storage
|
|
|
Hydrophilic vs hydrophobic on structure
|
Hydrophilic molecules readily interact with water molecules via dipole-dipole interactions or h-bonds. Hydrophobic molecules avoid water. Large biomolecules that have hydrophobic and hydrophillic arrange themselves accordingly
Proteins fold so that the hydrophobic sections are tucked inside. |
|
|
Membrane proteins
|
Integral - (embedded) vs peripheral (on surface)
Channel - transport specific ions across the membrane Carrier - Transport specific molecules across the membrane Receptors - signal transduction Anchor - Attach membrane to macromolecules |
|
|
Self assembly occurs because of.....
|
STERIC INFORMATION in the macromolecules: complementary shapes fit together to optimize hydrophilic interactions, hydrophobic interactions, and many weak interactions
|
|
|
Molecular machines
|
Examples: ribosomes and sarcomeres
1.Nucleotide binds to motor protein 2.Nucleotide hydrolyzes and releases energy 3.Energy causes changes in shape 4. Change is transmitted to nearby subunits |
|
|
Phases of signal transduction
|
1. Reception - signal molecule (ligand) binds to and activates receptor on membrane surface, causing transduction.
2. Transduction - a change in receptor shape 3. Response - inside the cell, a cascade of events that involves covalent modification of proteins and result in changes such as enzyme activity, gene expression, and motion |
|
|
Common features of prokaryotic cells
|
cell wall
plasma membrane circular DNA molecules No internal membrane-enclosed organelles -Pili and flagella |
|
|
Cell walls of prokaryotic cells (gram +/-)
|
Gram positive - thick peptidoglycan layer outisde plasma membrane
Gram negative - More complex cell walls: 1. glycocalyx - slime layer 2. outer membrane - LPS with porins 3. Periplasmic space - with peptidoglycans and proteins 4. Inner(plasma) membrane |
|
|
DNA of prokaryotic cells
|
Nucleoid - of chromosomes
Also has plasmids - small circles of DNA |
|
|
Eukaryotic plasma membrane structure
|
glycocalyx, receptors, extracellular matrix; cell cortex
|
|
|
Eukaryotic ER structure
|
Lumen/cisternal space
Rough - synthesis of membrane proteins and proteins for export Smooth - lipid synthesis, biotransformation Sarcoplasmic Reticulus - SER in striated muscle |
|
|
Eukaryotic Golgi Apparatus
|
packages and distributes cell products in compartments
Vesicles Called dictyosomes in plants |
|
|
Eukaryotic nucleolus
|
synthesis of ribosomal DNA
|
|
|
Function of mitochondria
|
aerobic metabolism - oxygen-dependent synthesis of ATP
Regulation of apoptosis |
|
|
Function of peroxisomes
|
break down peroxides (R-O-O-R)
|
|
|
Plant-only organelles
|
vacuole - contains acid hydrolases, like lysosomes
microfilaments cell wall (contains cellulose) Dictyosomes - like golgi peroxisomes - one kind is in leaves for photorespiration and GLYOXYSOMES, in germinating seeds convert lips to carbz |
|
|
Plastids are like...
|
MITOCHONDRIA.
Proplastids are plastid precursors: 1. Leucoplasts leukoplasts (storage) 2. Chromoplasts (pigment) - Chloroplasts - photosynth with grana, thylakoid lumen and membrane -stroma - like mitochondrial matrix |
|
|
Microtubules (3 types)
|
Structural support for long, thin cells; protein = tubulin
Microfilaments - cytoplasmic streaming and amoeboid movement; protein = actin Intermediate filaments - maintain cell shape under mechanical stress; various proteins; keratin |
|
|
How can H-bonds form?
|
occur between hydrogen that is attached to an oxygen/nitrogen and a lone pair of electrons (O,N,S)
|
|
|
How many hydrogen bonds can form
|
4/molecule
|
|
|
What does hydrogen bonding form
|
BP, MP, heat of vaporization, surface tension, heat capacity, and viscosity of water
|
|
|
What influences how strong van der Waals forces are?
|
How easily the atom is polarized
|
|
|
Types of vdW forces
|
Dipole-dipole
Dipole-induced dipole Induced-Induced - London dispersion. They are individually the weakest. |
|
|
Influence(s) of solvation spheres
|
1 - charge density. Smaller and more highly charged, larger the solvation sphere
2 - larger solvation spheres make spheres move more slowly |
|
|
What causes sol-gel transitions?
|
temp, matrix architecture, inclusion of solutes
|
|
|
Sol-gel transitions
|
gel-like properties of cytoplasm that result from polar surfaces of polymers forming highly structured solvation layers
Contibutes to cell movement and other functions including actin-binding proteins; amoeboid motion |
|
|
What causes hydrophobic interactions?
|
Water molecules maximize hydrogen bonds with other water molecules and minimize interaction with nonpolar moleucles. Attraction of nonpolar molecules contributes, but it's the force of water EXCLUDING nonpolar molecules that drives hydrophobic interactions
|
|
|
Cell membrane potential
|
Cytoplasmic side of cell is negatively charged due to amino acid R groups in proteins
|
|
|
Buffer formation
|
a weak acid and its conjugate base
|
|
|
Buffers are most effective when the pH is...
|
within a range of +/- 1 of the pKa
|
|
|
Open vs Closed vs Isolated Systems
|
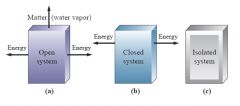
|
|
|
State vs process functions
|
Enthalpy entropy and free energy are state functions
heat and work are process functions |
|
|
How can systems spontaneously decrease entropy?
|
Surroundings become more disordered, overall disorder still has to increase (2nd Law of Thermo)
|
|
|
Delta G with a 0 up top
|
Free energy change at standard state: 25 Celsius, 1atm, 1M
|
|
|
Delta G with 01 up top
|
Free energy change at BIOCHEMICAL standard state - pH
|
|
|
Thermodynamics vs Kinetics
|
Thermo tells whether a reaction will be SPONTANEOUS/EXERGONIC (energy released)
Kinetics tells HOW FAST a reaction will go (involves enzymatic activity) |
|
|
Fibrous vs Globular Proteins
|
Fibrous - Function usually involves structural support
Globular - Function usually involves binding to LIGANDS or other macromolecules (COOPERATIVE BONDING) |
|
|
Biologically Active Amino Acids
|
Chemical Messengers
-Neurotrans - glycine, glutamate, GABA, serotonin, melatonin, -Hormones - thyroxine, indole acetic acid Precursors to complex N-molecules Metabolic intermediates |
|
|
Amino Acid Derivatives
|
formed thru carboxylation, hydroxylation, and phosphorylation
Example - serine, threonine and tyrosine all get phosphorylated (contain hydroxyl groups) |
|
|
Stereochemistry and amino acids
|
All amino acids except glycine contain a chiral carbon
All proteins contain L-Amino acids (Ds are supes rare) |
|
|
Typical pKa that amino acids ionize their COOH and NH2 groups
|
COOH around 2
NH2 around 9 |
|
|
Characteristics of Peptide Bonds
|
Partial double bond character - rigid and planar
Alpha carbon NEXT to carbonyl Psi - rotation around alpha carbon-Nitrogen bond Phi - rotation around alpha carbon- carbon bond |
|
|
Peptide/Protein sequence using termini
|
N to C terminus. IMPORTANT because order influences bonding which will influence pKa and schtuff
|
|
|
Functions of Proteins
|
Catalysis, structure, movement, defense, regulation, transport, storage, stress response
|
|
|
Protein shapes
|
Fibrous and Globular
|
|
|
Protein compositions
|
Simple vs Conjugated
Conjugated = simple + prosthetic group HOLOPROTEINS (CONJUGATED) HAVE (prosthetic groups) BUT (prosthetic groups are) ABSENT IN APOPROTEINS |
|
|
Characteristics of two types of secondary protein structures
|
Alpha helix - Hydrogen bonds are FOUR residues apart, R groups extend OUTWARD, incompatible with glycine, proline, and sequences with too many charged/bulky R groups
Beta sheet - Parallel or antiparallel, Adjacent chains H-bond, ANTIPARALLEL IS MORE STABLE because bonds are colinear and shorter |
|
|
Stabilizing Factors of Tertiary Protein Structure
|
Hydrophobic Interactions
Electrostatic Interactions Hydrogen Bonding Covalent Bonding (disulfide bridges) Hydration (hydration shells) |
|
|
Features of Tertiary Structure
|
1. Amino acids are far apart in primary structure, but get close and personal once folded.
2. Globular proteins are more compact 3. Large globular proteins contain DOMAINS, compact units with specific function. 4. Modular vs. Mosaic proteins - eukaryotes contain duplicate/imperfect copies of domains that are linked in series |
|
|
What holds quaternary structure in place?
|
noncovalent and
covalent bonds (disulfide bridges, desmosine and lysinoroleucine) MOST IMPORTANTLY, hydrophobic effect |
|
|
Function of IUPs
|
Intrinsically Unstructured Proteins
Regulation of signal transduction, transcription, translation, cell proliferation. They're super flexible and search for binding partners (to make them less fugly/disordered) |
|
|
Very basically, what causes denaturation?
|
Disruption of forces that stabilize 3-D structure
|
|
|
Denaturing Agents
|
strong acids
strong bases organic solvents detergents reducing agents (disulfide bonds) salt concentration (salting out) heavy metal ions temperature changes mechanical stress |
|
|
Types of motor proteins
|
1. Classical motors - myosins, kinesins, dyneins
2. Timing devices 3. Microprocessing switching devices 4. Assembly and disassembly factors |
|
|
Traditional Folding Model of Proteins
|
Amino acid side chain interactions force molecule into it's shape
Limitations: 1. Time constraints - seconds, not years 2. Complexity - LOTS of possible rotations |
|
|
Recent Protein Folding Discoveries
|
1. Secondary structure is formed super early
2. Hydrophobic interactions are super important 3. Larger polypeptides have partially folded intermediate structures 4. MOLECULAR CHAPERONES - Bind to unfolded/denatured proteins, protect them from incorrect interactions, assist in folding, and promote degradation when refolding isn't possible |
|
|
Most Molecular Chaperones are...
|
Heat Shock Proteins:
1. Hsp70s - bind to short hydrophobic segments in unfolded PPs to prevent aggregation. ATP hydrolysis releases PP, which is then passed to... 2. Hsp60s (chaparonins or Cpn60) - Mediate protein folding and release the PP upon ATP hydrolysis |
|
|
Fibrous Proteins
|
Structural Function - rodlike/sheetlike (regular secondary structure)
Examples - keratin (hair), collagen (tendons), silk, fibroin (silk) |
|
|
Example of the dynamic function of globular proteins
|
Globular proteins mainly function in binding to ligands or large biomolecules.
Myoglobin (cardiac/muscle) and Hemoglobin (red blood) Heme decreases affinity for oxygen and protects from irreverible oxidation of Fe2+ Fetal HbF has greater affinity for oxygen than maternal hemoglobin Myoglobin has greater affinity for oxygen than hemoglobin, will only give up oxygen when the concentration is super low |
|
|
Cooperative binding in hemoglobin is stimulated by...
|
taut state
oxygen binding High CO2 High BPG low pH |
|
|
Bohr Effect
|
Dissociation of O2 from hemoglobin is enhanced at low pH.
High CO2 concentration increases H+ concentration by reacting with water to form H+ and bicarbonate ions. H+ stabilizes deoxy form of hemoglobin |

