![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Biochemistry of Nitrogen |
N2 is chemically inert It takes a lot of E to break a triple bond but many organisms have enzymes to assist with this |
|
|
Overcoming E barrier for N2 (not on exam) |
Nitrogenase is an enzyme complex that uses ATP Passes e- to N2 and catalysis a reduction of N2 to NH3 Consumes about 16 ATP molecules |
|
|
2 enzymes in nitrogen assimilation (not on exam) |
1. Nitrate reductase: NO3–>NO2 Large solvable protein, contains Mo cofactor and e- from NADH 2. Nitrite reductase: NO2+6e-> NH4 Found in chloroplast in plants: e- come from ferredoxin I’m nonphotosynthetic microbes, e- comes from NADPH |
|
|
Anammox rxns (not on exam) |
Anaerobic ammonia oxidation Short cuts the nitrogen cycle |
|
|
Ammonia is incorporated through glutamate and glutamine |
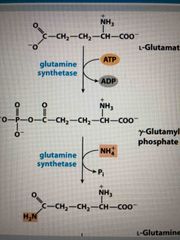
Glutamine is made from glutamate by glutamine synthetase (2 step process) Phosphorylation creates a good leaving group |
|
|
Adenylation of glutamine synthetase |
Adenylylation assist in inhibition |
|
|
Biosynthesis of amino acids |
Transaminations and rearrangements using pyridoxal phosphate (PLP) PLP comes from vitamin b6 Catalyezed by amidotransferases |
|
|
Amino acid synthesis |
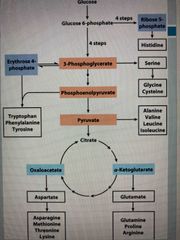
Source of N is Glu or Gln Derived from intermediates of: Glycolysis, citric acid cycle, pentose phosphate pathway |
|
|
All amino acids are derived from 7 precursors |

Citric acid cycle: 1. Alpha-ketoglutarate 2. Oxaloacetate Glycolysis: 3. Pyruvate 4. 3-phosphoglycerate 5. Phosphoenolpyruvate Pentose phosphate pathway: 6. Ribose 5-phosphate 7. Erythrose 4-phosphate |
|
|
Citric acid cycle makes Alpha-ketoglutarate which makes glutamate which can then make… |
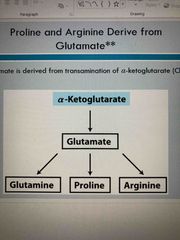
Glutamine Proline Arginine |
|
|
Glycolysis leads to 3-phosphoglycerate which can make.. |
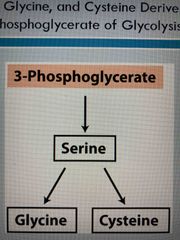
Serine and serine be converted to Glycine Cysteine |
|
|
Citric acid cycle and make oxaloacetate which then produces… |
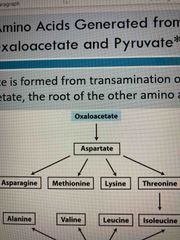
Aspartate which can make Asparagine Methionine Lysine Threonine |
|
|
Glycolysis generates pyruvate which makes.. |
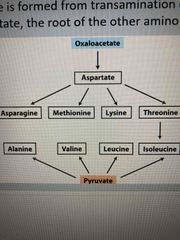
Alanine Valine Leucine Isoleucine |
|
|
Glycolysis generates phosphoenolpyruvate and PPP generates erythrose 4-phosphate which combine to give you… |
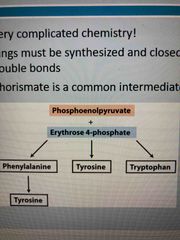
Phenylalanine (can also become tyrosine) Tyrosine Tryptophan These are aromatics and complicated to produce They all share a chorismate intermediate |
|
|
PPPgenerates ribose 5-phosphate which generates… |
Histidine |
|
|
Several pathways share 5-phosphoribosyl-1-pyrophosphate (PRPP) as an intermediate |
Synthesized from ribose 5-phosphate of PPP via ribose phosphate pyrophosphokinase (highly regulated enzyme) |
|
|
Regulation of AA biosynthesis |
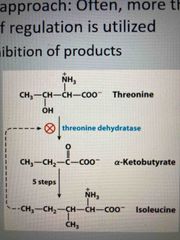
Multilayered approach, often more than one mechanism of regulation is used Feedback inhibition of products |
|
|
Use of Isozymes in regulation |
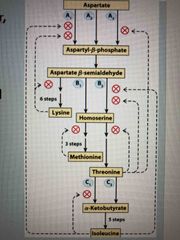
Isozymes are regulated by different factors Allows for production of AA as needed ie: Asp can lead to Lys, Met, Thr, and Ile |
|
|
Porphyrins |
Makes up heme of hemoglobin, cytochromes, and myoglobin Precursors are glycine or glutamate In higher animals, use glycine with succinyl-CoA |
|
|
Defects in heme biosynthesis |
Most animals synthesize own heme but mutations or misregulation of enzymes can lead to porphyrias (precursors accumulate in erythrocytes, body fluids, and liver) ie: accumulation of uroporphyrinogen I |
|
|
Uroporphyrinogen I accumulation |
Urine is discolored (pink to purple depending on light/heat exposure) Teeth are red under UV light Skin is sensitive to UV light Craving for heme Reason for vampire myths |
|
|
Heme is source of bile pigments |
Heme is degraded to bilirubin in 2 steps: 1. Biliverdin (green) seen in bruise 2. Converts to bilirubin (yellow) which make urine yellow |
|
|
Bilirubin accumulation |
Causes jaundice Results from: impaired liver (liver cancer, hep) Blocked bile secretion (gallstones, pancreatic cancer) Insufficient enzyme that breaks down bilirubin (occurs in infant) which can be treated by exposing to UV light
|
|
|
NTs |
Are made by decarboxylation of AAs |
|
|
Arg makes nitric oxide in body |
. |
|
|
Nucleotide biosynthesis |
Nucleotides can be synthesiszed in 2 ways: 1. De novo-“from the beginning” made from AAs, ribose-5-phosphate, CO2, and NH3 2. Salvaged- from RNA, DNA, and cofactor degradation |
|
|
De novo |

Bases synthesized while attached to ribose Glutamate provides most AA groups Glycine is precursor for purines Asp is precursor for pyrimidines Must continually synthesize nucleotides and amount may affect rate of transcription and replication |
|
|
De novo biosynthesis |
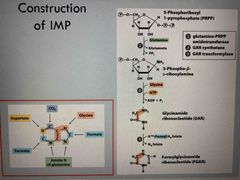
Starts with 5-phophoribosyl 1-pyrophosphate (PRPP) with glutamine Purine ring builds up following the addition of 3 carbons from glycine The first intermediate with a full purine ring is inosinate (IMP) |
|
|
Inosinate can make either AMP or GMP |
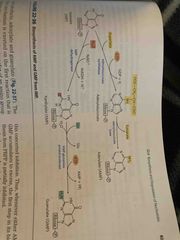
From the inosinate intermediate… You can add GTP and make AMP Or add ATP and make GMP |
|
|
De novo synthesis Of pyrimidine nucleotides |
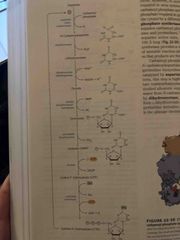
Unlike purine, it starts out making a ring (orotate) and attaches to ribose 5-phosphate via PRPP Forms orotidylate which is then decarboxylation to form uridylate (UMP) UMP is phosphorylated to UTP Animation occurs to convert to CTP |
|
|
Ribonucleotides are precursors to deoxyribonucleotides |
2 C-OH bond is reduced to 2-H bond without activating the carbon (catalyzes by ribonucleotide reductase) Mechanism: 2 H atoms are donated by NADPH and carried by proteins thioridoxin or glutaredoxin |
|
|
Ribonucleotide reductase involves free radicals |
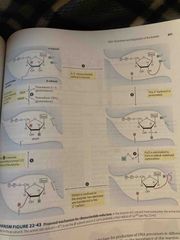
Most forms of enzyme have 2 catalytic/regulatory subunits and 2 radical-generating subunits 3’ ribonucleotide radical forms 2’-OH is protonated to help eliminate H2O and form a radical-stabilized carbocation e- are transferred to the 2’ C |
|
|
Folic acid deficiency leads to reduced thymidylate synthesis |
Reduced thymidylate synthesis causes uracil to be placed into DNA Repair mechanisms remove the uracil creating strand breaks that affect structure and function of DNA (associated with cancer heart disease and neurological impairment) |
|
|
Catabolism of purines |
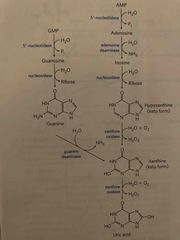
1. De phosphorylation by 5’-nucleotidase 2. Deamination and hydrolysis of ribose produces xanthine 3. Hypoxia thins and xanthine are oxidized into uric acid by xanthine oxidase |
|
|
Excess uric acid in gout |
Painful joints Affects males Under excretion of urate or overconsumption of fructose Treated with avoidance of purine rich foods (seafood and liver) or avoidance of fructose and xanthine inhibitor allopurinol |
|
|
Catabolism of pyrimidines |
Leads to NH4 and urea Can produce intermediates of citric acid cycle |
|
|
Purine and pyrimidine bases are recycled by salvage pathways |
Free bases released in metabolism are reused The brain is dependent on salvage pathways Lack of hypoxanthine-guanine phosphoribosyltransferace leads to Lesch-nyhan syndrome with neuro impairment and finger/toe biting |

