![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
360 Cards in this Set
- Front
- Back
|
What is the most primitive bilaterally symmetric animal?
|
Planaria.
|
|
|
What's so special about planaria?
|
They're really good at regenerating.
|
|
|
How are planaria able to regenerate?
|
They have a population of cells called neoblasts that are involved in regeneration.
|
|
|
What family are planaria a part of?
|
Platyhelminthes.
|
|
|
What are 3 general parts of a planarian?
|
1. Eyes with photoreceptors
2. Branched gut system 3. Pharynx on the ventral side where they eat and poop through the same tube. Ew. |
|
|
What happens if you cut a planarian in half?
|
The top part will make a posterior blastema and the bottom part will make an anterior blastema and you will generate two planarians.
|
|
|
blastema:
|
regeneration bud.
|
|
|
The anterior portion of the planarian is...
|
the head region.
|
|
|
The posterior portion of the planarian is...
|
the bottom end region.
|
|
|
The only proliferating cells in planarians are...
|
neoblasts.
|
|
|
How do you experimentally show that neoblasts give rise to regenerating parts?
|
You label neoblasts with BrDU before you amputate them. The blastema contains many BrDU labeled cells.
|
|
|
What's wrong with irradiated planarians?
|
They can't regenerate.
|
|
|
What happens if you inject purified neoblasts into an irradiated planarian?
|
You can restore its ability to regenerate.
|
|
|
What three factors does a planarian lose when it's irradiated?
|
Neoblasts, smedwi-1, smedwi-2.
|
|
|
Which smedwi seems to be the most important?
|
smedwi-2 seems to be more important for regeneration.
|
|
|
What does smedwi-2 do?
|
It's essential for regeneration and homeostasis.
|
|
|
What happens if you use smedwi-2 RNAi in planarians?
|
They fail to form blastemas - the phenotype is similar to if the planarians were irradiated.
|
|
|
What does a radiated planarian head look like?
|
It gets all shrunken in; it's kinda gross.
|
|
|
What does a smedwi-2 RNAi planarian head look like?
|
It looks similar to the irradiated planarian head. It head starts to get shrunken in which is creepy.
|
|
|
What does smedwi-2 RNAi do to the neoblasts?
|
The neoblasts can't function normally. You can see in the experiment that with smedwi-2 RNAi, there is no tissue surrounding the neoblasts.
|
|
|
What happens if you put a wild type planarian chunk into an irradiated planarian?
|
You can save the whole planarian and they look all happy.
|
|
|
What happens if you put an irradiated chunk into an irradiated planarian?
|
It just dies and degrades after around 29 days.
|
|
|
What's another name for smedwi-2?
|
Smed-piwi. Thanks for making me remember yet another name.
|
|
|
You irradiate a planarian and then you take a wild type chunk and stain for smed-piwi. What happens?
|
You see the cells from the chunk repopulate the lethally irradiated cells of the host. It makes the planarian look like a murderous demon, but it saves the planarian.
|
|
|
If you're really mean, you irradiated the planarian and then decapitate it. Then you attach the irradiated headless thing to a section of wild type and stain for smed-piwi. What do you see?
|
You see the smed-piwi begin to migrate up toward the decapitated portion. The smed-piwi doesn't move up if you haven't cut off the head. This is an example of directional migration.
|
|
|
How do you experiment with directional migration in planarians?
|
Irradiate a planarian and cut off its head. Now attach the irradiated section to a wild-type section with a smed-piwi marker. You'll see the cells migrating up to make a new head.
|
|
|
What kind of cells do you see when regenerating a decapitated planarian?
|
agat-1
|
|
|
What's the pathway for cell differentiation in planarians?
|
neoblasts --> prog-1 --> agat-1
|
|
|
What are the stem cells of the planarian?
|
Neoblasts
|
|
|
How do you make the Janus phenotype in planarians?
|
You have to knock down beta catenin in the planarian and you'll end up getting a head at the place you chopped off.
|
|
|
What does beta catenin do in the planarian?
|
It is required for blastema AP polarity.
|
|
|
How do you get rid of beta catenin in the planarian?
|
RNAi.
|
|
|
How does beta catenin relate to the axial levels of the planarian?
|
If you deplete beta-catenin with RNAi, you can cut a multiple different places and you'll still get a head that forms.
|
|
|
What's the six headed monster?
|
It's this planarian, that mad scientists depleted beta-catenin in using RNAi. Then they chopped all over the planarian and got a new head at ever cut.
|
|
|
What happens if you deplete beta-catenin and then chop off just the most anterior portion of the planarian?
|
They still regenerate heads right by their heads.
|
|
|
Based on RNAi studies, how is beta-catenin functioning in the planarian?
|
It probably doesn't simply inhibit anterior formation. It probably promotes posterior fates instead on a gradient level.
|
|
|
What's the insitu Wnt signaling for planarian?
|
Beta-catenin is involved in Wnt signaling so if you stain for 6 different Wnts, you'll see them in different places throughout the planarian. Now you cut the planarian in three parts (head, trunk, tail) and stain for the Wnts again. You will see the specific Wnts in the same place in each of the sections.
|
|
|
What's another way you mess with beta-catenin in the planarian?
|
You could inhibit beta-catenin half way in the planarian and look at the fate of the planarian.
|
|
|
What is a planarian stem cell marker?
|
Smedwi-2 (aka Smed-piwi)
|
|
|
How do amputated planarian pieces deterime AP identity?
|
Through beta-catenin dependent mechanisms.
|
|
|
How do you perform RNAi on a planarian?
|
You take homogenized liver and infect it with e coli which has a plasmid that makes dsRNA. The planarian eats the liver and dsRNA which will break down the specific mRNA you want.
|
|
|
Why can't you use RNAi on humans through feeding them dsRNA?
|
Humans see dsRNA as a virus and will attack the dsRNA.
|
|
|
Urodeles can regenerate...
|
limbs and hearts.
|
|
|
Zebrafish can regenerate...
|
hearts.
|
|
|
How do you prove experimentally that zebrafish can regenerate their hearts?
|
Cut part of their heart off and do insitu hybridization for cardiac myosin heave chain and a dye indicating fibrin. It'll grow back.
|
|
|
How can you see if cardiomyocytes come from cardiomyocytes?
|
Use a beta-actin2:RSG zebrafish with CRE ER. Under normal conditions, the beta actin will glow red. Then after you injure the heart, when you add tamoxifen, the CRE will induce the gene to make GFP. The new cardiomyocytes glow green so they come from cardiomyocytes.
|
|
|
What happens to mature cardiomyocytes after injury?
|
They dedifferentiate into early embryonic heart cells again and express embryonic cardiac differentiation markers like Nkx 2.5, tbx5.
|
|
|
What three factors are found in the zebrafish regenerating heart?
|
Mef2 and Myosin Heavy Chain (MHC) are in the cardiomyocytes and DAPI is in the nuclei.
|
|
|
2 factors in the regenerating cardiomyocytes:
|
Mef2 and MHC
|
|
|
Factor in the regerating nuclei of the zebrafish heart:
|
DAPI
|
|
|
What factor shows activation during zebrafish heart injury?
|
GATA4. That's what is labeled with GFP.
|
|
|
During zebrafish cardiac injury, what factor do you see in the injury site?
|
GATA-4
|
|
|
How can you determine the contribution of GATA4 descendants in the heart?
|
Use GATA4 CRE ER. When you injure the zebrafish heart, the GFP-expressing cells migrate down to the injury site and de-differentiate.
|
|
|
How much does the human heart increase from birth to adulthood?
|
30-50 fold
|
|
|
How are most human heart cells reacting as the heart grows?
|
Via hypertrophy instead of cell division.
|
|
|
How do you test the origin of heart cells in humans?
|
Between WWII and 1963, there was a lot of C14 in the air. C14 was incorporated into dividing cells and can be used to date the age of cells. You find that people born before WWII have higher C14 levels in their cardiomyocytes compared to when they were born. Similarly, people born after WWII have lower levels than they were born with. This tells you that cardiomyocytes do proliferate, but slowly.
|
|
|
Turnover rate of cardiomyocytes at age 20:
|
1%
|
|
|
Turnover rate of cardiomyocytes at age 75:
|
0.4%
|
|
|
What's the difference between a proximal amputation and a distal amputation on a salamander?
|
The proximal amputation goes more slowly, but you ultimately end up with the same regenerated limb.
|
|
|
In urodeles, the blastema has a cap made of...
|
apical epidermal cells.
|
|
|
What are the 5 stages of urodele limb regeneration?
|
1. Amputation
2. Healing 3. Dedifferentiation 4. Cone stage 5. Palette stage 6. Notch stage 7. Digit stage |
|
|
What happens in the dedifferentiation stage of regeneration?
|
Proliferation of blastema cells and the presence of the apical epidermal cap.
|
|
|
What happens in the palette stage of regeneration?
|
The dedifferentiated cells redifferentiate.
|
|
|
What are the 3 assays you can do to look at PD identity of blastemal cells?
|
1. Confrontation assay
2. Grafted blastema assay 3. Level of Blastema assay |
|
|
What's the confrontation assay?
|
If you stick a proximal blastema by a distal blastema, the proximal blastema will engulf the distal blastema.
|
|
|
What's the grafted blastema assay?
|
If you cut off a salamander arm, it will make a blastema. Now cut off that blastema and stick it somewhere on the arm stub. The more proximal you put it, the longer the two arms will be. The more distal the two blastemas and you'll just have autopods.
|
|
|
What is the level of the blastema assay?
|
The tissue of the limb stump will regenerate to the level of the blastema. If you switch the blastemas of a proximal and a distal amputation, you'll get one really short and one really long limb. This is because the proximal blastema gets attached to the proximal and becomes super proximal. The other limb is fine.
|
|
|
Why would a proximal blastema attached to a proximal stump become super proximal?
|
Structures from the shoulder to the wrist arise by growth from the proximal partner. The blastema itself can't proximalize.
|
|
|
How can you make a 3-armed salamander monster?
|
You sew up its arm into its stomach so it looks like it's doing "I'm a little teapot." Then you cut the teapot arm in half and it grows 2 arms, one out of its stomach!
|
|
|
Why does making an "I'm a little teapot" salamander monster work?
|
Distal regeneration occurs even when the cut surface is proximal facing. For some reason...
|
|
|
What does retinoic acid do to a stub?
|
It reprograms the blastema. In the presence of retinoic acid, the salamander forms a completely new limb because retinoic acid is a proximal signal.
|
|
|
What happens if you treat a wrist blastema with retinoic acid?
|
It makes a completely new limb. So you get an super long limb.
|
|
|
What happens if progenitors for regeneration are lineage-restricted?
|
You find out that there are no stem cells. Skeletal cells make more skeletal cells.
|
|
|
What can the blastema regenerate?
|
It has a "memory" of where the amputation occurred. It can regenerate all tissues distal, but not proximal from the point of amputation.
|
|
|
What do you see from the regenerated limbs of axolotls?
|
They appear to be derived from multiple progenitor cells that represent major tissue lineages rather than a single pluripotent cell, ie there are no stem cells.
|
|
|
What factor resets the memory of a blastema?
|
Retinoic acid.
|
|
|
What are the two main stages of sex determination?
|
1. Germ cell development
2. Sex determination (dose compensation) |
|
|
Early in development, how do germ cells differentiate themselves form somatic cells (2 ways)?
|
1. They're sequestered very early in development.
2. They are in some aspects immortal. |
|
|
Where are germ cells specified?
|
In the periphery of embryos.
|
|
|
How do germ cells get to the gonads?
|
They have to migrate.
|
|
|
3 stages of germ cell development:
|
1. Specification
2. Migration 3. Differentiation |
|
|
What are P-granules?
|
Germ line-specific cells from the earliest stages in c elegans that act as instructors of germ line development.
|
|
|
What are the 2 roles of p-granules?
|
1. Instructors of germ line development
2. bind RNA and promote germ-cell specific trxn. |
|
|
What is Pie-1?
|
A trxn factor that prevents the phosphorylation of RNA pol II and blocks trxn in germ cells during development.
|
|
|
What's wrong with Pie-1 mutants?
|
They have embryonic genes in the P-cell.
|
|
|
What happens if you put Pie-1 in somatic cells?
|
It causes repression of somatic transcripts.
|
|
|
Where are primordial germ cells induced?
|
In the posterior region of the embryo immediately next to the extraembryonic ectoderm.
|
|
|
How many primordial germ cells are made from induction?
|
20
|
|
|
When does specification of the PGC occur?
|
E 7.5
|
|
|
What are PGCs?
|
primordial germ cells
|
|
|
What does Blimp1 do?
|
It's the earliest marker of PGCs and is a trxnal repressor.
|
|
|
When does migration of PGCs occur?
|
E 9.5
|
|
|
When does reprogramming of PGCs occur?
|
E 11.5
|
|
|
What day does mammalian sex differentiation occur?
|
E 12.5
|
|
|
What happens to Blimp1 mutants? 2 things.
|
Their PGCs fail to migrate. You can stain the PGCs with alkaline phosphatase and see they don't migrate. They also fail to repress somatic cell markers (Hox genes).
|
|
|
How do you perform single cell cDNA analysis of PGC cells?
|
Isolate the RNA, transcribe to cDNA with RT, assay by PCR and run on a gel to look for specific genes, probably via Western blotting.
|
|
|
What are pole cells?
|
Drosophila germ cell progenitors. They start at the tip and migrate to the midgut where they attach and align with the gonadal mesoderm.
|
|
|
What are the 4 steps of pole cell migration?
|
1. Attachment to endoderm and migration through the midgut
2. Attachment to mesoderm 3. Alignment with gonadal mesoderm 4. Two streams of migrating cells. |
|
|
Which direction do the drosophila germs cells migrate in the midgut?
|
Dorsally before splitting into two streams.
|
|
|
What are Wunen genes?
|
Wunen and Wunen-2 genes are redundant lipid phosphate phosphatases that act as chemorepellants expressed in the ventral midgut.
|
|
|
What do lipid phosphate phosphatases do?
|
They remove extracellular phospholipid substrates.
|
|
|
What do Wunen phosphatases do?
|
They guide PGCs by repelling them.
|
|
|
Why do PGCs only migrate through the endoderm dorsally?
|
They are repelled by ventral Wunen.
|
|
|
Why do PGCs split into two streams?
|
They are repelled by additional Wunen expressed in the CNS.
|
|
|
Wunen is necessary for...
|
proper lateral migration.
|
|
|
What happens if you knock out wunen but not wunen-2?
|
Nothing because the phosphatases are redundant for each other.
|
|
|
What happens to Wunen; Wunen-2 double mutants?
|
Their PGCs are unable to migrate laterally when they are in the middle of the embryo. They start going every which way.
|
|
|
How can you visualize PGC migration?
|
You can use a PGC-specific antibody.
|
|
|
How can you rescue a Wunen;wunen-2 double mutant?
|
CNS-specific expression in embryos can partially rescue gonad expression.
|
|
|
How do mice PGCs migrate?
|
They move into the endoderm (hindgut) and then migrate into the body wall.
|
|
|
What happens with mice PGCs at E 9?
|
The cells are motile, but do not leave the hindgut epithelium.
|
|
|
What are the 3 locations that mice PGCs migrate through?
|
hindgut --> body wall --> genital ridges
|
|
|
What are the two forms of X-inactivation in mammals?
|
1. During early cleavage stages, the paternal X-chromosome is inactivated in all cells. It stays inactivated in the extraembryonic tissue but is reactivated in the embryo proper.
2. In the embryo, then one of the two X-chromosomes is randomly inactivated in each cell. |
|
|
How are tortoishell cats the way they are?
|
During X-chromosome inactivation and as the embryo grows, one of the two x-chromosomes will randomly inactivate in each cell. For that reason, some cells will have a black fate and some will have an orange fate.
|
|
|
What is x-chromosome inactivation in mice?
|
The mouse has Xist RNA which is a 17kb non-coding RNA produced only from the inactive X (Xi). It blankets the entire chromosome and is thought to initiate chromosome-wide silencing.
|
|
|
What is Xist RNA?
|
It's a 17kb non-coding RNA which is produced only by the inactive X.
|
|
|
Where is Xist RNA seen?
|
In mice and only in females. You have to have more than 1 X chromosome.
|
|
|
How is Xist trxn regulated?
|
Xist binds to a specific region on the X-chromosome (the Xic). Xist is regulated by binding to an antisense transcript on the same transcript called Tsix. Tsix is required for an active X.
|
|
|
Which chromosome produces Xist RNA?
|
Only the inactive X (Xi). It negatively regulates itself.
|
|
|
How does an active X chromosome keep from getting shut off by Xist?
|
The active chromosome produces Tsix which creates dsRNA with Xist and then gets degraded.
|
|
|
What exactly does Xist do to the inactive chromosome?
|
It stabilizes and recruits repressive histone markers.
|
|
|
Where are germ cells usually specified?
|
In the periphery of the embryos (pole cells, P-granules)
|
|
|
How do germ cells differ from somatic cells during development?
|
They keep a low profile and transcriptionally repress somatic cell fates.
|
|
|
How are gonads formed?
|
The migration of the germ cells and their combining to somatic cells.
|
|
|
What is Sxl?
|
It is an RNA binding protein and is the master sex regulator in drosophila.
|
|
|
What does early Sxl trxn stimulate?
|
Female drosophila sex development
|
|
|
Which sex is default in drosophila?
|
Male
|
|
|
What does Sxl control?
|
Dosage compensation.
|
|
|
What does Sxl control?
|
Dosage compensation
|
|
|
Sxl is only functional in...
|
females.
|
|
|
How do drosophila compensate for XX in females?
|
They overexpress X proteins in males. This is opposite of mammals which use bar bodies.
|
|
|
Sxl and Tra don't work in males because...
|
it has an extra exon which tells the protein to terminate so that it becomes nonfunctional.
|
|
|
What is the pathway for Sxl?
|
Sxl --> Tra --> Dsx
|
|
|
What does DSX-M do?
|
It is the protein that makes male drosophila.
|
|
|
Promoters of Sxl:
|
Pe and Pm.
|
|
|
Where do Pe and Pm occur?
|
The Sxl promoters both occur in both sexes. Pe occurs only in females.
|
|
|
What is the difference between the Pe and Pm transcript?
|
The Pe transcript is functional, but the Pm requires pre-existing Sxl to be spliced properly to make functional Sxl.
|
|
|
XCE =
|
X chromosome counting elements
|
|
|
What does active transformer (tra) do?
|
Makes female-specific doublesex (Dsx)
|
|
|
What does Dsx do?
|
Processes Fruitless
|
|
|
What is fruitless in charge of?
|
Sexual behavior
|
|
|
Which drosophila trxn factor is the most important for sex determination?
|
Dsx.
|
|
|
What are 4 downstream effects of female Dsx?
|
1. female-specific growth genital disc
2. yolk proteins 3. spermathecal ducts 4. female pigmentation |
|
|
4 downstream effects of male Dsx?
|
1. Male-specific growth genital disc
2. Paragonia 3. Clasper 4. Male pigmentation |
|
|
Sxl inhibits...
|
Msl2
|
|
|
Msl2 is not inhibited in...
|
males
|
|
|
What happens if Sxl isn't activated early?
|
You get male junk.
|
|
|
Pe is only active for...
|
30-40 minutes
|
|
|
What does Msl2 do?
|
It opens the chromatin so that trxn is increased in the male x chromosome.
|
|
|
What are the two different pathways in drosophila?
|
1. Plumbing
2. Sexual preference |
|
|
Sexual behavior of wt female drosophila:
|
No courting behavior
|
|
|
Sexual behavior of wt male drosophila:
|
1. Chase the female
2. Play a song 3. Lick her |
|
|
What is essential for male sex behavior?
|
Correct splicing for Fruitless
|
|
|
What happens to males lacking Fru sex-specific transcripts?
|
They have normal sexual differentiation, but are completely sterile because they don't persue females.
|
|
|
What happens to Fru(male) females?
|
She persues wt females.
|
|
|
What is the primary sex determination of mammals?
|
Creation of gonads
|
|
|
The gonad is initially...
|
bipotential; it can become either ovary or testis
|
|
|
What is mammalian secondary sex determination?
|
The phenotype outside the gonads (duct systems, external genitalia)
|
|
|
at 4 weeks in human gonad development, you have...
|
indifferent gonads. You have a wolffian duct and mesonephric ridge. You also have a genital ridge.
|
|
|
At 6 weeks human gonad development, you have...
|
You have Mullerian duct development and germ cells in the genital ridge.
|
|
|
8 weeks testis development:
|
1. Wolffian duct --> vans deferens
2. Mullerian duct 3. Testis develops from genital ridge |
|
|
8 weeks ovarian development:
|
1. Wolffian duct degrades
2. Mullerian duct 3. Surface epithelium on genital ridge |
|
|
16 weeks testis development:
|
1. Mullerian duct degrading
2. Testis cords have formed. |
|
|
20 weeks ovarian development:
|
1. Ovarian cortex and follicles form
2. Wolffian duct degrading |
|
|
What is SRY?
|
A trxn factor and is the mammalian sex determining gene
|
|
|
What happens if a female has sry?
|
It becomes an XX male - male genitalia, but no functional sperm.
|
|
|
2 hormones made from testis:
|
1. AMH (anti-mullerian hormone)
2. Terosterone |
|
|
What does AMH do?
|
Causes Mullerian duct regression
|
|
|
What does testosterone do in development?
|
Causes differentiation of Wolffian duct into internal male genitalia (epididymis and vas deferens).
|
|
|
Internal male genitalia:
|
1. epididymis
2. vas deferens |
|
|
Two kinds of cells in the testis:
|
1. Sertoli cells
2. Leydig cells |
|
|
Sertoli cells make...
|
AMH
|
|
|
Leydig cells make...
|
Testosterone
|
|
|
2 cells in ovary:
|
1. Granulosa cells
2. Thecal cells |
|
|
The mullerian duct creates...
|
female internal genitalia (uterus, oviducts, cervix, upper vagina)
|
|
|
Female external genitalia comes from...
|
genital tubercle, urogenital sinus
|
|
|
Testosterone turns on...
|
DHT
|
|
|
DHT controls...
|
Penis, prostate, scrotum
|
|
|
Wolffian duct controls...
|
Male internal genitalia.
|
|
|
What happens to XX mice lacking FoxL2?
|
They develop male gonads. The follicle cells begin to look like Sertoli cells and secrete testosterone (Leydig cells) You can convert eggs into sperm. They eggs apoptose.
|
|
|
Evidence against female default status:
|
FoxL2 is needed to generate and maintain female gonads.
|
|
|
How can you prove that FoxL2 is needed to maintain female gonads?
|
Use CRE ER and wait to add tamoxifen. The females will start producing testosterone when FoxL2 gets turned off.
|
|
|
When FoxL2 turns off...
|
Sox9 gets turned on.
|
|
|
Two mammalian factors that turn the undifferentiated gonad into testis:
|
1. Sry
2. Sox9 |
|
|
What happens to guevedoces in Dominican Republic?
|
Their bodies dont produce DHT so they start lives out with female genitalia and then produce outer male genitalia during puberty. They can have kids.
|
|
|
2 factors needed for external genitalia:
|
1. testosterone
2. DHT |
|
|
Androgen Insensitivity Syndrome:
|
Individuals lack testosterone receptor. The individual can be XY, but has female secondary sex characteristics. They have internal male sex characteristics though.
|
|
|
Sex determination in turtles:
|
It depends on the temperature that the eggs incubate. 31* (hotter) become ovaries. 26* (cooler) become testis. Many turtles, some lizards, and all crocodiles are determined by temperature.
|
|
|
What does the Hh inibiting drug do?
|
It can reduce your tumors for a bit, but they come back worse later.
|
|
|
Inappropriate expression of signaling molecules after birth causes...
|
cancer.
|
|
|
Inappropriate Hedgehog signaling in the brain causes...
|
medulloblastoma
|
|
|
Basal cell carcinoma can be caused by...
|
inappropriate Hh signaling.
|
|
|
How do neurons in the cerebellum differentiate?
|
The first neuroblasts migrate to form the external granular layer. Granule neurons are produced in the External Granule cell layer and migrate back towards the lumen to form the granule cell layer. The EGL is gradually depleted.
|
|
|
Where to the granule neurons go?
|
To the external granule layer (EGL).
|
|
|
6 layers of the cerebellum:
|
1. Ventricular zone
2. Intermediate zone 3. Granule cell layer 4. Purkinje cell layer 5. Marginal zone 6. External granule cell layer |
|
|
Where do neuroblasts go from the ventricular zone?
|
They migrate to form Purkinje cell layer.
|
|
|
Why are Purkinje cells so cool?
|
They're huge and are the sole motor output of the cerebellum.
|
|
|
How do cells in the cerebellum proliferate?
|
Hh signaling
|
|
|
Hh signaling pathway:
|
Shh binds to --> Patched receptor --> inhibits Smo
|
|
|
Most common pediatric brain tumor:
|
medulloblastomas
|
|
|
If you have a Ptch mutation...
|
Smoothened never gets turned off so Hh is always on.
|
|
|
Where does Shh signaling come from in the cerebellum?
|
It comes from the PCL and EGL cells respond to it. When you stain for it, Shh seems to be in the EGL layer.
|
|
|
What does Shh signaling do in the cerebellum?
|
It causes proliferation of GCPs in the cerebellar slice cultures.
|
|
|
Mitogen:
|
growth factor
|
|
|
Mitogen in the cerebellum:
|
Shh
|
|
|
What happens if you don't have Anti-Shh in the cerebellum?
|
GCP don't proliferate. They have a much smaller layer of EGL. Purkinje cells are fine though because they secrete Shh.
|
|
|
Medulloblastoma is rare in...
|
adults
|
|
|
What is the drug they used for that patient with medulloblastoma?
|
It's a small molecule inhibitor of smoothened called GDC-0449.
|
|
|
What is the challenge of working with animal models for cancer?
|
When you take cells out of an animal, they start to behave differently.
|
|
|
What happens to Ptch +/- mice?
|
50% develop medulloblastomas.
|
|
|
What happens if you're Ptch +/-;p53+/-?
|
You get an insane amount of cancer.
|
|
|
How many genes are directly activated by Shh signaling?
|
500 target genes!
|
|
|
How does ChIP work to find Gli-activated proteins?
|
You get a Gli generally with a Flag Tag and you have the Gli flag tag bind to unknown sequences and then you use a restriction digest and chop it all up. Next throw in some beads that have an antibody to the flag tag. The antibody will bind to any pieces that have Gli protein attached. Stick in a magnet and wash - the antibody will attach to the magnet and everything else will wash away. Finally, you release the proteins and DNA and sequence the DNA to find out the Gli targets.
|
|
|
How do you find the Gli binding sites in medulloblastomas?
|
You have to breed a Patch1+/- mouse with a tagged Gli mouse line (RosaGli1Flag).
|
|
|
What kind of cancer can you lessen with Smoothened inhibitors?
|
You can help treat basal cell carcinoma and it works ok on medulloblastomas.
|
|
|
Wnt signaling plays a role in what cancers?
|
Breast, colorectal, etc.
|
|
|
Pluripotent:
|
(of an immature or stem cell) capable of giving rise to several different cell types.
|
|
|
What kind of stem cells make muscel?
|
Satellite cells
|
|
|
Ovarian stem cells are...
|
Presophiles
|
|
|
Embryonic stem cells are...
|
pluripotent.
|
|
|
What happens to TCF4-/- mice have gut defects?
|
They're born, but die in 24hrs. They fail to maintain proliferative compartments in the intestinal epithelium. At birth, the entire epithelium is composed of non-dividing, differentiated cells.
|
|
|
What is TCF4 essential for?
|
Maintaing gut homeostasis.
|
|
|
The absorptive epithelium of the small intestine is ordered into...
|
vili and crypts.
|
|
|
What do vili do?
|
They allow for the increase in surface area for absorbing nutrients.
|
|
|
What's the life cycle of individual epithelial cells?
|
Less than a week.
|
|
|
Stem cells in crypts make how many cells a day?
|
200/crypt/day in a mouse.
|
|
|
What does Lgr5 do?
|
It marks small intestinal stem cells.
|
|
|
Where is Lgr5 expression?
|
Only in a subset of cells at the base of the crypt.
|
|
|
What's the Wnt target gene in small intestine?
|
Lgr5
|
|
|
Paneth cells:
|
One of the principle cell types in the small intestine
|
|
|
2 roles of paneth cells:
|
1. Emit microbial agents descended from stem cells.
2. Are always proliferating |
|
|
What's the difference between paneth and quiescent muscle cells?
|
Paneth are always proliferating and quiescent cells only proliferate in damage.
|
|
|
How can you visualize the lgr5 cells in a mouse?
|
You make a mouse with an Lgr5-GFP Cre ER allele. Once you activate with tamoxifen, after day 1, you see GFP in the base. Over time though, it will start to cover the whole vili.
|
|
|
What do paneth cells secrete?
|
Wnt proteins
|
|
|
What does Lgr5 amplify?
|
Wnt signaling response in stem cells.
|
|
|
How do you grow mini-gut organoids if you're weird like that?
|
You grow in vitro from a single Lgf5-expressing cell and it will get 5 cryps around the main lumen and will become functional!
|
|
|
What's wrong with TCF4-/- in relation to Wnt signaling?
|
They can't activate beta-catenin.
|
|
|
What is FAP?
|
An APC protein discovered via FAP disease in which patients get colon cancer.
|
|
|
When does FAP become cancerous?
|
FAP leads to pollups, but when the cells lose p53 and K-ras, that's when they become cancerous.
|
|
|
How do you give a mouse colon cancer?
|
You mess up its APC alleles and it will proliferate rapidly and give the mouse colon cancer.
|
|
|
What is nuclear reprogramming:
|
The ability to change the fate of a cell by making a switch in nuclear gene expression.
|
|
|
3 reasons nuclear reprogramming is of interest:
|
1. Identifying how reprogramming takes place can help us understand how cell differentiation and specialized gene expression is usually maintained.
2. Could be the first step in cell-replacement therapy 3. It enables the culture of lines of cells to be analyzed for therapeutic drugs. |
|
|
How did they make Dolly?
|
They transplated the nuclei of cultured mammary gland cells from a sheep into enucleated sheep eggs.
|
|
|
What is nuclear reprogramming and how does it relate to cloning?
|
It's the switch in gene expression of one cell type to that of another cell type. For cloning, it means reprogramming the nucleus of a differentiated cell into a pluripotent cell.
|
|
|
What are the steps of the early cloning experiment?
|
1. Remove chromosomes and spindle from the cell of an animal pole
2. Extract and lyse the donor cell 3. Stick the donar nucleus into the enucleated cell 4. PUt the somatic cell nucleus in an activated egg 5. You get a frog (yay!) |
|
|
What happens when you take nuclei later in development?
|
The percent of embryos that develop normally decreases exponentially.
|
|
|
What did Sir John Gurdon do?
|
He took the nucleus from an albino frog and put it in a brown frog and would keep taking it out putting it in other enucleated cells and eventually was able to reprogram the cell.
|
|
|
How did they make Dolly according to the slides?
|
They took an egg from an oocyte donor. They removed other cells from another sheep and grew them and then stuck them in the enucleated egg. The egg and cell fused and an embryo was cultured for 7 days. A blastocyst formed and then they put it in the surrogate mom and voila Dolly.
|
|
|
What's the controversy with Dolly?
|
Some people did think it was really cloning and it was a really rare event - 1/434 injections.
|
|
|
What is OMP?
|
Marker for differentiated olfactory sensory neurons.
|
|
|
How do you find out the origin of olfactory cells?
|
You use a CRE that only expressed in terminal olfactory neurons by sticking it to OMP.
|
|
|
Describe the experiment that makes a mouse out of olfactory sensory neurons.
|
Rudolph Jaenisch's group showed that either terminally differentiated olfactory sensory neurons or lymphocytes can be used to generate mice. You put the nuclei in ES cells, culture a blastocyst and then stick it in a surrogate.
|
|
|
Why can you reprogram an oocyte?
|
It probably still has some maternal factors. It's under prolongued miotic arrest. The egg can be unstable the egg has no nucleus for a while during the experiment. But it works in an oocyte.
|
|
|
What is the first embryonic cell cycle?
|
1. After fertilization, the reprogramming factors are initially in the cytoplasm are in the nucleus.
2. You can treat zygotes with nocodazole which results in mitotic arrest until the drug's removed. 3. During this arrest, NEB has already happened so reprogramming can last longer. MG-132 inhibits proteasome, prevents degradation of cyclin B. |
|
|
MG-132:
|
Inhibits proteosome and prevents degradation of cyclin B (blocks metaphase to anaphase)
|
|
|
Nocodazole:
|
Inhibits spindle formation and results in mitotic arrest.
|
|
|
Could zygotes be used instead of oocytes for Reprogramming?
|
They froze the zygote and used it to clone an ES cell, but the zygotes were inviable. Lots of cells from fertilization and polyspermy - it causes messed up zygotes.
|
|
|
What development stage is more ethical in in vitro fertilization treatment?
|
The author Egli argued that it's more ethical to use defective zygotes because using a human zygote has the capacity to form an embryo. So you should use one with multiple nuclei.
|
|
|
How did they make the human ES cells in the new data?
|
They took skin fibroblasts and human blastocysts.
|
|
|
What was the goal when they were making iPS cells?
|
To try to reprogram cells without having to use eggs.
|
|
|
Fbx15?
|
It is turned on in ES cells, but off in somatic cells.
|
|
|
Fbx15 is not essential...
|
for ES cells.
|
|
|
How did Yamanaka make iPS cells?
|
They infected mouse embryonic fibroblast viruses with 24 candidate genes possibly required for generating stem cell fates and ended up with some colonies.
|
|
|
How did Yamanaka see which cells were iPS cells?
|
He gave the Fbx15 Neomycin resistance so that as long as the cells were iPS cells, they would produce neo R on a neo plate and survive.
|
|
|
What are 4 critical factors for iPS cells?
|
1. c-Myc
2. Klf4 3. Sox2 4. Oct 3/4 |
|
|
What happens if you try to make iPS cells without Sox2?
|
You can still make colonies, but they look really weird.
|
|
|
What do iPS colonies look like?
|
ES cells
|
|
|
What can you use iPS cells for?
|
You can make chimeric embryos with GFP expression and can make various tissue types in adult chimeras.
|
|
|
problems with iPS cells:
|
1. Retroviuses permanently integrate into the genome. So that's bad because the viruses could reactivate.
2. iPS cells aren't very efficient (10^-4 of the population) 3. Two of the Yamanka factors (C-Myc and Klf-4) are oncogenes and could make a tumor. |
|
|
Which Yamanaka factors are oncogenes?
|
c-Myc and Klf4.
|
|
|
What are the most narrowed down two critical Yamanaka factors?
|
Oct4 and Sox2
|
|
|
iPSCs make all three...
|
embryonic germ layers: endoderm, mesoderm, ectoderm
|
|
|
Are iPSCs just as good as ES cells?
|
Yeah.
|
|
|
What happens if you don't culture ES cells?
|
They start to make cardiomyocytes and neurons?
|
|
|
How do you differentiate ESCs and IPSCs into motor neurons?
|
You culture ES cells with different levels of RA or Shh/RA and you get different progenitors.
|
|
|
What happens if you culture ES cells with RA?
|
You get dorsal neural progenitors.
|
|
|
What happens if you culture ES cells with Shh and RA?
|
You get ventral neural progenitors.
|
|
|
How do ESCs and IPSCs compare when trying to give them neural fates?
|
They differentiate into motor neurons at similar rates.
|
|
|
How could you make IPSCs for people with Type 1 diabetes?
|
You could take skin fibroblast biopsies, generate human iPS cell lines which could then be differentiated into beta cells.
|
|
|
What's the biggest problem with IPSCs?
|
They have troublesome memories. They seem to retain imprinting form what they used to be.
|
|
|
What are the worst IPSCs from?
|
Skin fibroblasts neural cells.
|
|
|
It's harder to make IPSCs in what kind of cells?
|
Older cells.
|
|
|
IPSCs are better at making ____ than ___
|
bone; blood
|
|
|
What's causing the memory of these IPSCs?
|
Genes have methylation markers that are maintained throughout life of the cell.
|
|
|
How can you rescue IPSC neural cells from remembering?
|
You can treat them with 5-Azac
|
|
|
5-Azacytidine:
|
Drug inhibiting DNA methylation
|
|
|
Dor et al used a pulse-chase approach to label beta cells in adult mice. Describe their experimental approach and conclusions. What would have been the result if a non-beta cell progenitor gave rise to other beta cells?
|
Dor et al crossed a mouse line expressing a beta-cell specific, tamoxifen inducible Cre with a Cre reporter line (irreversibly expresses Alkaline phosphatase upon Cre-mediated activation). Without tamoxifen, the CreER protein is sequestered in the cytoplasm and is therefore unable to activate the reporter gene (which requires nuclear localization of Cre). They treated adult mice with an intermediate dose of tamoxifen that allowed for activation of the Cre reporter in ~25% of beta cells when assayed a few days post-induction. They saw no difference in the percent of labeled cells – even a year later. B/c the half-life of beta cells is only ~3 months, and because there is a tremendous increase in the total number of beta cells over this time, they conclude that beta cells are giving rise to new beta cells. If, instead, they had seen a progressive reduction in the percentage of labeled beta cells, it would have suggested that a non-beta cell progenitor population was present.
|
|
|
Zhou et al 2008 described an experimental approach for generating beta cells, showing that forcibly expressing three transcription factors in exocrine cells transformed them from exocrine cells to endocrine fates (including beta cells). The data we showed in class stopped short of proving that the ‘new’ beta cells were derived from non-beta cells. Describe an experiment and result that would directly demonstrate that these new cells were descended from amacrine cells?
|
You would need a tamoxifen inducible Cre line that is only active in the exocrine cells and a Cre reporter. If you injected with tamoxifen at the same time when you infected the mice with the transcription factor cocktail, cells that expressed endocrine markers (such as insulin) and GFP+ (indicating viral infection) should also express b-galactosidase (assuming RosaLacZ is the Cre reporter).
|
|
|
What is the key transcription factor that separates exocrine and endocrine pancreatic lineages?
|
Neurogenin 3 (Ngn3) is required for the formation of endocrine lineages.
|
|
|
When you graft a distal (wrist-level) axolotl blastema to another animal that has a proximal limb amputation, replacing the original host blastema, you still form a normal limb. Which portion will be derived from the host, and which portion from the blastema?
|
The region up to the wrist will be derived from the host (intercalary regeneration). The region distal to the wrist level will be derived from the blastema.
|
|
|
When you graft a distal (wrist-level) axolotl blastema to another animal that has a proximal limb amputation, replacing the original host blastema, you still form a normal limb. Which portion will be derived from the host, and which portion from the blastema?
-The region up to the wrist will be derived from the host (intercalary regeneration). The region distal to the wrist level will be derived from the blastema. Repeat the experiment in #1 except that the donor animal is first treated with high doses of retinoic acid for several days. What happens? |
High levels of retinoic acid will completely proximalize the blastema. When grafted, it will then form a complete limb after the proximal stump. This will result in a limb with extra appendages.
|
|
|
Injury to zebrafish ventricles results in the initial activation of genes throughout the entire region of a specific tissue in the heart. Which tissue?
|
A sub-population of GATA4+ cardiomyocytes adjacent to the epicardium.
|
|
|
What is the functional effect of mammalian cells lacking Xist?
|
There will be no inactivation of X-chromosomes and there will therefore be a lethal increase in X-chromosome genes.
|
|
|
What is Xist? How is it controlled? How does it inactivate X-chromosome transcription?
|
Xist is a long non-coding RNA that binds to the X-chromosome at a specific region (Xic) and randomly initiates inactivation of one of the X-chromosomes. Xist is only transcribed and presence on the inactive X-chromosome. Prior to X-inactivation, Xist is transcribed at low levels on both X-chromosomes, but is inhibited by being bound by an antisense non-coding transcript called Tsix. After initiating inactivation of one X-chromosome, Xist transcription is highly upregulated, eventually binding to sites along the entire X-chromosome. Xist facilitates the recruitment of histone modification enzymes that are markers of silenced regions, and also bind along the inactive X-chromosome.
|
|
|
What are the differences in how PIE-1 and Blimp1 repress somatic gene transcription in germline progenitor cells?
|
While both negatively regulate transcription, PIE-1 represses all Pol2 mediated zygotic transcription (by inhibiting transcriptional elongation of Pol2) while Blimp1 is a sequence specific transcriptional repressor that doesn't turn off all somatic genes.
|
|
|
How is germline specification different in C. elegans versus mice?
|
Germ cells are autonomously specified in C. elegans (sequestration of P-granules and PIE-1) while in mice they are induced (by BMPS) in the extraembryonic tissue.
|
|
|
The mechanism by which primordial germ cells (PGCs) migrate from the hindgut endoderm into the genital ridges to populate the two future gonads is not understood in mice. Propose a testable hypothesis for how PGCs might split into two streams as they migrate out of the hindgut in mice based on your knowledge of the process in Drosophila. Your hypothesis should include specific molecule(s) as well as where you hypothesize they should be expressed, and how you might alter/remove them.
|
Wunen and Wunen-2 mediate midline repulsion of PGCs as they migrate out of the Drosophila hindgut, thereby allowing them to form two discrete populations that migrate laterally to populate the two gonads. Based on this, a reasonable hypothesis is that they might play an analogous role in mice. If so, they might be expressed in a midline structure (CNS or more likely mesodermal). If so, you would need to determine if there are mouse orthologues (by searching the mouse genome for genes with similar structure - or the literature; as described on the discussion board there are functional orthologues of Wunens in mammals that can even substitute for Wunen activity in flies) and where they are expressed (by in situ hybridization). Then, you would need to generate null mice, and look for defects in germ cell migration….
|
|
|
Why do you think Sex lethal (Sxl) is in fact lethal when mutated in female flies?
|
Because females will then have twice as much transcription from genes on their X-chromosome.
|
|
|
How is Sxl null mutant female similar or different to a null mutant in Transformer (Tra)?
|
Sxl lethal is upstream of Tra; both have the same effect that in their absence, they will generate male-specific isoforms of the sex determination transcription factor Doublesex (Dsx). They are different because Sxl has an additional role in inhibiting Msl genes, thereby preventing amplification of transcription in female cells. Thus, transformer only effects the sex determination pathway and not the doseage compensation pathway. Because of this, Tra mutants are not lethal, and XX females will display male morphology and male courting behavior (since they will have a male isoform of Fruitless).
|
|
|
Why don't XX mice expressing an Sry transgene produce functional sperm?
|
Sry is sufficient to initiate the male sexual development pathway. However, additional proteins necessary for making sperm are also present on the Y-chromosome.
|
|
|
What happens to the Mullerian duct in males? In females?
|
It degenerates in males, where Sertoli cells (which are present in the fetal testis, secrete anti-mullerian hormone that promotes its apoptosis. In females it forms the oviduct and contributes to the internal genitalia.
|
|
|
Which fetal cells secrete testosterone?
|
The Leydig cells (along with the Sertoli cells the two major somatic copmonents of the testis.
|
|
|
What is the difference between individuals with mutations in the testosterone receptor vs. mutations in synthesizing DHT?
|
If the receptor is mutated, the testes will form. However, the testosterone secreted by the testes cannot be sensed, leading to the development of female secondary sexual characteristics. Individuals with DHT mutations still form internal genital structures, and in the case of the guevedoces, will form full male secondary structures at puberty (presumably because there is a surge of testosterone).
|
|
|
Speculate on the phenotype of a female fly with normal sex determination genes except for a mutated version of Fruitless that can only generate a male isoform.
|
The fly will be a fertile female fly but will exhibit male mating behavior (e.g. will court females).
|
|
|
You generate embryonic flies with a deletion in the Pe (early) promoter for Sxl. What is the phenotype for females, and why?
|
Sxl will be transcribed later from the Pm promoter, but this will not encode for a functional protein. In females, this will result in the activation of Msl2, leading to a lethal overdose of transcription from the X chromosome.
|
|
|
What is the progenitor population that gives rise to granule cells in the cerebellum? Is this population retained in adults?
|
The external granule layer (EGL). This layer is not retained past a relatively early postnatal period (2 years in humans) – not present in adults.
|
|
|
If you used an EGL-specific Cre to remove Shh, what would happen?
|
Nothing! The EGL responds to (does not produce) Shh secreted by the Purkinjie Cells.
|
|
|
Elevated levels of Wnt signaling have been implicated in colon cancer. Propose a plausible mouse model system that would allow you to study this WITHOUT perturbing Wnt signaling during development or in other tissues (besides the colon).
|
Need some type of strategy that allows for the elevation of Wnt signaling in a Cre-specific fashion. One strategy would be an inducible Cre that would activate Wnt signaling in the colon (by perturbing or deleting a negative regulator or activating a positve factor. For example inducing a dominant negative in a Cre-dependent fashion…or, conditionally deleting a negative regulator (Axin) in adult colon tissue.
|
|
|
How do the experiments outlined in Egli et al differ from classic nuclear transfer?
|
Instead of transferring nuclei into enucleated oocytes, they transferred chromosomes into zygotes that have had their chromosomes removed. Importantly, the zygotes do not have their nuclei removed. Instead, they are frozen in metaphase when the nuclear envelope has broken down (and all nuclear reprogramming components are therefore retained in the zygote).
|
|
|
How did Egli et al. freeze their cells in metaphase? Why was this necessary?
|
They froze cells by arresting them in metaphase with the drug nocodazole (this destabilized microtubules and prevents the mitotic spindle from forming). They then removed Nocodazole, allowing the spindles to form completely synchronously in all their cells, and added MG-132, which inhibits the proteasome-based degradation of Cyclin B. This second drug freezes them at a stage when the mitotic spindles have formed. This was necessary because this is the only stage at which chromosomes can be viewed and removed under the microscope without adding toxic dyes and viewing under UV.
|
|
|
Why does dysregulated, activated Wnt signaling cause intestinal cancer?
|
Lgr5-expressing stem cells require Wnt signaling for their maintenance (they get this from their neighboring Paneth cells). If there is more Wnt signaling, there will be more stem cells. These will form a poly that is, at this stage, benign. With an additional mutation in another gene (P53, K-ras), these cells will transform into an adenocarcinoma.
|
|
|
Instead of nuclear transfer, you decide it will be far easier to generate an induced pluripotent stem cell line (iPS cell) from cardiomyocytes and then use this to clone mice. How would you generate an iPS cell line from a cardiomyocyte?
|
Infect primary cardiomyocytes with the Yamanaka factors (Sox2, Oct4, Klf4, Myc - don't actually have to use Myc in newer approaches). Look for ES cell-like colonies growing on the dish over time (here you would be greatly aided by the fact that differentiated cardiomyocytes undergo little or no proliferation).
|
|
|
Describe the positive selection screen used by Takahashi and Yamanaka to isolate the 4 "Yamanaka" factors.
|
They used Fbx15, a gene that is only expressed in ES cells (but not essential) to drive neomycin resistance. They then isolated fibroblasts from mice that were Fbx15::Neo-R. These cells are differentiated and Fbx15 is therefore not expressed; thus these cells are neomycin sensitive. They then infected these cells with 24 viruses expressing 24 candidate genes that they hypothesized might be involved in making pluripotent cells. When all 24 were added, they obtained several neomycin-resistant colonies that closely resembled ES cells. They then sequentially removed 1 factor from the 24, infecting pools of 23 factors. This narrowed down the number of candidates to 10. They then showed that only 4 of these were required to make iPS cells.
|
|
|
What are enhancers?
|
Non-coding elements that can be located either close or far away from the genes.
|
|
|
What do enhancers do?
|
They can modulate activity regardless of their orientation.
|
|
|
What are enhancers bound by?
|
Clusters of transcription factors.
|
|
|
What are promoters?
|
Docking sites for RNA pol II.
|
|
|
How can you experimentally ID enhancers?
|
Candidate enhancers are inserted into a piece of DNA that is then injected into embryos to generate transgenics. You can then use in situ hybridization to visualize where the enhancer and gene are.
|
|
|
Transgenics:
|
The manipulation of a gene to visualize the gene or enhancer.
|
|
|
What are 4 regions of a typical developmental gene?
|
1. Enhancer regions
2. Promoter regions 3. Transcribed regions 4. Coding regions |
|
|
What happens to primary RNA transcripts?
|
They are processed (spliced) into mature mRNA and exported to the cytoplasm.
|
|
|
Two things that can occur to cytoplasm mRNAs?
|
1. They can be stored.
2. They can be actively translated into proteins. |
|
|
Bauplan:
|
The most basic anatomical structure for the vertebrate embryo.
|
|
|
Draw the basic body plan.
|
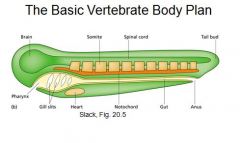
|
|
|
3 things ectoderm turns into:
|
1. Epidermal cells of skin
2. Neurons of the brain 3. Pigment cells |
|
|
5 things mesoderm turns into:
|
1. Notochord
2. Bone tissue 3. Tubule cell of the kidney 4. Red blood cells 5. Facial muscle |
|
|
3 things endoderm turns into:
|
1. Stomach cells
2. Thyroid cells 3. Lung cells |
|
|
What are von Baer's 4 laws?
|
1. The general features of a large group of animals appear earlier in development than do specialized features of a specific group.
2. Less general characters develop from the more general. 3. The embryo of a given species, instead of passing through the adult stages of lower animals, departs more and more from them. 4. The early embryo of a higher animal is never like a lower animal, but only like its early embryo. |
|
|
The phylotypic stage:
|
The stage of development at which all members of a taxon (especially insects and vertebrates) show the maximum morphological similarity.
|
|
|
What is the bauplan?
|
The virtual embryo that has the generalized feature of a large group of animals.
|
|
|
What is the major goal of evolutionary developmental biology?
|
To uncover previously invisible homologies that contribute to the body plan.
|
|
|
What are the 2 different nerves that innerve the middle ear?
|
1. The trigeminal nerve
2. The Facial nerve |
|
|
What does the Trigeminal nerve innervate?
|
The tensor tympani muscle of the Malleus
|
|
|
What does the Facial nerve innervate?
|
The Stapedius muscle of the Stapes.
|
|
|
3 things derived from the 1st pharyngeal arch:
|
1. upper and lower jaws
2. Malleus and Incus 3. Trigeminal ganglion |
|
|
3 things derived from the 2nd pharyngeal arch:
|
1. Facial muscles
2. Portions of the throat 3. Stapes, facial nerve |
|
|
Why are two tiny muscles in the middle ear innervated by completely different nerves?
|
The trigeminal nerve innervates the Malleus because both are derived from the first arch. The facial nerve innervates the stapes because both are derived from the second pharyngeal arch.
|
|
|
Compare mammal, reptile, and fish ear bones.
|
Fish have no middle ear bones
Reptiles have 1 ear bone - the stapes. Mammales have 3 ear bones. |
|
|
How was it shown that leg length in dogs is controlled by few genes?
|
Cross a tall and short dog in the parent generation and you'll see short and long in the F2 which have unique combinations of the two original heights. It's in a 3:1 ratio.
|
|
|
What two features are controlled by relatively few genes in dogs?
|
1. Leg length
2. Upper vs. lower jaw |
|
|
How are marien and freshwater sticklebacks different?
|
Marine stickleback have pelvic armor and freshwater don't.
|
|
|
Whats the experiment you can do with sticklebacks?
|
Cross male and female from each species to make F1 hybrids. Then make an F2 generation. Look at the microstellite markers. 40% of variation can be attributed to one locus.
|
|
|
What does Pitx1 do?
|
It's a limb specific gene in mice. The mutation in mice is lethal, but embryos have smaller hindlimbs.
|
|
|
How is Pitx1 related to sticklebacks?
|
Both marine and freshwater sticklebacks have the Pitx1 wt gene, but it's expressed in the mouth AND the pelvic plate for marine sticklebacks.
|
|
|
What happens when Pax6 is misexpressed in drosophila?
|
They get ectopic eyes all over the place - like on their knees.
|
|
|
What happens to Pax6+/- humans?
|
They have aniridia which means that their iris is messed up.
|
|
|
Are eyes an example of convergent or divergent evolution?
|
Convergent.
|
|
|
Whats the genetic basis for diversity?
|
Recombination during meiosis.
|
|
|
How does meiosis differ from mitosis?
|
It has 2 rounds of cell division with only one round of DNA replication.
|
|
|
Oocytes:
|
Eggs
|
|
|
How much bigger are mammalian oocytes from a somatic cell?
|
100-500 fold difference.
|
|
|
Why are oocytes so big?
|
They have to store more nutrients, mRNAs, mitrochondria, etc.
|
|
|
Vertebrate oocytes are arrested in...
|
meiotic prophase.
|
|
|
How much DNA do oocytes have?
|
Twice as much so 4 gene copies.
|
|
|
7 things that are amplified in oocytes:
|
1. mitochondria (100K)
2. RNA polymerase (60-100K) 3. DNA polymerase (100K) 4. Ribosomes (200K) 5. tRNA (10K) 6. Histones (15K) 7. dNTPs (2500) |
|
|
What is the cell support in drosophila oocytes?
|
The cell undergoes 4 incomplete divisions to make 15 nurse cells and 1 oocyte. The oocyte is surrounded by follicle cells.
|
|
|
How long will oocytes remain in arrest in humans?
|
12-50 years
|
|
|
What are the components of the secondary oocyte?
|
Oocyte and PB1
|
|
|
What are the components of an ovum?
|
Egg, PB1, and PB2.
|
|
|
When do your number of oocytes majorly drop?
|
Like right after birth.
|

