![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
241 Cards in this Set
- Front
- Back
|
adenosine triphosphate or ATP |
- the molecule that provides energy in a form that all cells can readily use to perform the work of the cell - often called universal currency |
|
|
Organisms can be classified according to their _______ and _______ sources |
- energy and carbon |
|
|
phototrophs |
- organisms that capture energy from sunlight EX: plants = uses the energy of sunlight to convert carbon dioxide and water into sugar and O2 |
|
|
chemotrophs |
- derive their energy directly from organic molecules such as glucose EX = animals; obtaining organic molecules through ingesting other animals. |
|
|
what are the two sources of carbon for chemotrophs and phototrophs? |
autotrophs ( carbon from CO2) and heterotrophs ( carbon from organic compounds) |
|
|
autotrophs |
" self-feeders" because they make their own organic sources of carbon - convert carbon dioxide into glucose |
|
|
heterotrophs |
" other feeders" - obtain their carbon from organic molecules synthesized by other organisms, they eat other organisms |
|
|
photoheterotrophs |
some micro-organisms that gain energy from sunlight, however obtain their carbon from performed organic molecules |
|
|
chemautotrophs |
some microorganisms extract energy from inorganic sources but build their own organic molecules - found in extreme environments - deep-sea vents |
|
|
metabolism |
- a set of chemical reactions that sustain life - the building and breaking down of sugars such as glucose, and the harnessing and release of energy in the process driven by chemical reactions in the cell - divided into two branches: catabolism and anabolism |
|
|
catabolism |
is the set of chemical reactions that break down molecules into smaller units and in the process, produce ATP - one of the branches of the metabolism |
|
|
anabolism |
set of chemical reactions that build molecules from smaller units and require an input of energy, usually in the form if ATP - one of the branches of the metabolism |
|
|
energy of a system |
- systems capacity to work |
|
|
kinetic energy |
the energy of motion - most objects process kinetic energy because they perform work, resulting in the movement of themselves and the surrounding matter.
|
|
|
what are the forms of kinetic energy |
- thermal - light - electricity |
|
|
potential energy |
- an energy that is possessed by an immobile object, or stored energy - depends on the structure of the object, or its position relative to it surroundings - released by an objects change in structure or position |
|
|
chemical energy is a form of ___________ energy? |
potential |
|
|
what is ATP composed of? |
adensoine = - base adensoine - five-carbon sugar ribose this is attached to: triphosphate |
|
|
where is ATP held? explain!!! |
- held in the bonds connecting the phosphate groups. - phosphate groups are negatively charged and tend to repel eachother - these bonds store potential energy, so when they are broken they release energy into the cell |
|
|
what are the chemical relatives of ATP? |
ADP ( adenosine diposphate) and AMP ( adenosine monophosphate |
|
|
the first law of thermodynamics |
- law of the conservation of energy - states that the universe contains a constant amount of energy, that can neither be created to destroyed, only converted one form yo another |
|
|
the second law of thermodynamics |
- disorder tends to increase - when energy changes form, the total remans the constant, however the energy available to go work decreases. |
|
|
entropy |
the degree of disorder ( second law of thermodynamics) |
|
|
gibbs free energy |
the amount of energy available to do work |
|
|
exergonic |
reactions with a negative change in G that release energy and proceed spontaneously |
|
|
endergonic |
reactions with a positive change in G and are not spontaneous |
|
|
enthalpy |
total amount of energy ( H ) |
|
|
entropy ( S ) |
the degree of disorder, and is multipated by absolute temp |
|
|
absolute temperature ( T ) |
- measured in kelvin - entropy is multiplied by this because temp influences the movement of molecules = hence the degree of disorder |
|
|
total amount of energy (H) = |
energy available to do work ( G) + energy loss to entropy ( TS) |
|
|
G = ( equation ) |
H - TS |
|
|
change in G = ( equation) |
change in heat - T x change in S |
|
|
chemical reaction |
- atoms retain their identity during a chemical reaction as bonds are broken and new bonds are formed to yield new molecules - |
|
|
catabolic reaction |
- negative change in G and releases energy in the form go ATP
|
|
|
anabolic reaction |
- have a positive change in G and require an input of energy often in the form of ATP |
|
|
the hydrolysis of ATP releases ________? |
energy |
|
|
hydrolysis reaction |
a chemical reaction which a water molecule to spilt into a protein and a hydroxyl group. - often break down polymers into their subunits and in the process one product gains a protein and the other gains a hydroxyl group |
|
|
the reaction of ATP with water is an ___________ reaction that ________ energy |
- exergonic - releases |
|
|
non-spontaneous reactions are often coupled to _______________ reactions? |
spontaneous |
|
|
energetic coupling |
- a spontaneous reaction drives a non-spontaneous one, which provides the thermodynamic driving force of a non-spontaneous biochemical reaction - the most common type of this reaction is the hydrolysis |
|
|
1- A ----> B 2- B ----> C what is their change in G |
1- change in G is more then 0 ( energy consumed) 2- change in G is less then 0 (energy released ) |
|
|
what do coupled reactions involve |
- they share a phosphate group, which is transferred between ATP and other molecules in the reaction |
|
|
enzymes |
- proteins that act as catalysts - increase the rate of chemical reactions - they are highly specific, acting on only certain reactions and catalyzing only some reactions, meaning they determine which chemical reactions take place
|
|
|
enzymes reduce the __________ _________ of a chemical reaction? |
activation energy |
|
|
transition state |
- intermediate stage between reactants and products - highly unstable and therefore has a large amount of free energy - in all chemical reactions the reactant adopts at least one transition state before conversion into products - the highest few energy value corresponds to the transition state |
|
|
activation energy ( E ) |
- the energy input necessary to reach the transition state |
|
|
an enzyme catalyzed reaction |
- an enzyme accelerated reaction by lowering the activation energy - the change in G is the same with and without an enzyme |
|
|
enzymes form a ______ with reactants and products |
complex, allowing them to not be consumed in the reaction |
|
|
substrate |
- in an uncatalyzed reaction, a reactant, that is converted to a product |
|
|
active site |
- is the portion of the enzyme that binds substrate and converts it to a product - in this site, the enzyme and substrate form both transient covalent bonds and together these interactions stabilize the transition state and decrease the activation energy |
|
|
why are enzymes so large? |
the large size of many enzymes is required at lard in part to bring the catalytic amino acids into very specific positions in the active site of the folded enzyme. |
|
|
the formation of the enzyme-substrate complex promotes the _____? |
the reaction between 2 substates by aligning their reactive chemical groups and limiting their motion relative to each other |
|
|
how do we know that enzymes form complexes with substrates? ( experiment ) |
- savante Arrhenius - 1888 catalase forms a complex with hydrogen peroxide - used spectral analysis, different molecules absorb different wavelengths of light. by analyzing the patterns pf absorption peaks as the reaction proceeded, hence he could determine whether or not an complex was formed. |
|
|
inhibitors |
- decrease the activity of enzymes - quite common, synthesized naturally by many plants and animals as a defense against predators. - are many medications that are used to treat decreases |
|
|
activators |
increase the activity of enzymes |
|
|
Irreversible inhibitors |
usually form covalent binds with enzymes and irreversibly inactivate them |
|
|
reversible inhibitors |
form weak bonds with enzymes and easily dissociate from them - can act competitively or non-competitively with enzymes |
|
|
competitive inhibitors |
bind to the active site of enzyme and prevent the binding of the substrate - they compete with the substrate for the active site of the enzyme. - structurally similar to the substrate - reduce the affinity of the enzyme for the substate |
|
|
non-competitive inhibitors |
- different structure from that of the substrate and bind to the enzyme at a site different from the active site - slows down the reaction by altering the shape of the enzyme and reducing the activity |
|
|
allosteric enzymes in the cep are regulated by __________ and _________? |
- activators and inhibitors |
|
|
negative feedback |
describes the effect in which the final product of a biochemical pathway inhibits the 1 step. the process in which a stimulus acts on a sense that communicates with an effector, producing a response that opposes the initial stimulus . - is used to maintain steady conditions or homeostasis |
|
|
negative regulation |
the process in which a regulatory molecule must bind to the DNA at a site near the gene to prevent transcription |
|
|
allosteric enzyme |
ex: threonine dehydratase - change their shape or conformation on ending to a substrate, activator or inhibitor and this change in shape in turn influences the activity of the enzyme, either activating or inhibiting it. |
|
|
cofactor |
- a substance that associates with an enzyme and plays a key role in its function.
|
|
|
metallic cofactor |
EX: ion, Mg, cobalt, Zn - bind to diverse proteins, including those used in DNA synthesis, nitrogen metabolism and the transport of electrons for cellular respiration and photosynthesis |
|
|
cellular respiration |
- a series of chemical reactions that convert the energy stored in fuel molecules into a chemical form that cab be readily used in cells |
|
|
glycolysis |
the breakdown of glucose to make pyruvate |
|
|
is type of reaction is cellular respiration? |
one of the major sets of catabolic reactions in a cell |
|
|
aerobic respiration |
- cellular respiration that happens in the presence of oxygen - most organisms, including some bacteria |
|
|
anaerobic respiration |
- cellular respiration that happens without the presence of oxygen |
|
|
what is stage one of aerobic cellular respiration? |
- glucose, fatty acids or amino acids are partially broken down and a modest amount of energy is released - EX: the breakdown of glucose to make pyruvate, known as glycolysis |
|
|
what is stage two of aerobic cellular respiration? |
- the pyruvate is converted to another molecule called acetyl-coenzyme A ( acetyl-CoA) and carbon oxide is produced |
|
|
acetyl-coenzyme A |
the substance that pyruvate is converted into through the second step of cellular respiration |
|
|
what is stage three of aerobic cellular respiration |
- the citric acid cycle - acetyl-CoA is broken down and more carbon dioxide is released |
|
|
electron carries |
- this is one of the energy carry used in the stages 1-3 - molecules that store and transfer energy in the form of "high-energy" or "excited" electrons. |
|
|
what is stage 4 of the aerobic cellular respiration |
oxidative phosphorylation - in this series of reactions, electron carries generated in stages 1-3 donate their high energy elections to an electron transport chain ( respiratory chain ) |
|
|
what are the two types of chemical energy storing molecules? |
ATP and electron carries |
|
|
electron transport chain |
- transfer electrons along a series of membrane-associated proteins to a final electron acceptor and harness the energy of electrons to produce a large amount of ATP. - extract energy from full molecules as well as in photosynthesis to exact energy form sunlight |
|
|
in the aerobic respiration what is the final electron acceptor? in step 4 |
- oxygen, it is consumed and water is produced |
|
|
in eukaryotes where does glycolysis? where does the citric acid cycle and oxidative phosphorylation take place? |
- glycolysis = cytoplasm - citric acid cycle and oxidation = mitochondria |
|
|
what is the electron chain made up of? |
- proteins associated with the inner mitochondria membrane |
|
|
endosymbiotic theory, and where does the reactions take place? electron transport? |
- in bacteria these reactions take place in the cytoplasm and the electron transport chain is located in the plasma membrane - this lead to the theory that mitochondria were once a free-living bacteria but now are unable to live outside their host eukaryotic cell. |
|
|
what happens in detail in stages 1 and 2 through cellular respiration? |
fuel molecules are partially broken down, producing ATP and electron carriers
|
|
|
what is happening in detail in stages 3 |
- fuel molecules and fully broken down, producing ATP and electron carries |
|
|
what is happening in more detail in stage 4 |
- electron carries donate electrons to the electron transport chain, leading to the synthesis of ATP |
|
|
cellular respiration involves a series of _________ reactions? |
redox |
|
|
oxidation-reduction reactions ( redox reactions) |
- often used to store or release chemical energy - biological systems |
|
|
oxidation |
- loss of electrons |
|
|
reduction |
is the gain of electrons |
|
|
reduction reaction |
electrons are transferred from one molecule to another so that one molecule loses electrons and one molecule gains those electrons |
|
|
oxidation is an ________ of electron density |
decrease - this is because electrons are not completely transferred between molecules |
|
|
reduction is an __________ of electron density |
increase - this is because electrons are not completely transferred between molecules |
|
|
electron sharing in covalent bonds |
electrons are more likely to be found near the more electronegative atom in a bond - the one with less electronegative is oxidized and the one with more is reduced |
|
|
electron acceotor |
an atom that gains electrons, is an oxidizing agent |
|
|
electron donor |
an atom that give away its electrons and therefore a reducing agent |
|
|
chemical energy is stored in reduced molecules such as __________ and _________ |
carbohydrates and lipids |
|
|
why do reduced molecules, such as carbohydrates and lipids, have high potential energy? |
- with how the atoms of these molecules share electrons - this is result of the fact that the electrons are far from the nucleus of the atoms |
|
|
what are the important types of electron carriers? (4) |
- oxidation form: NAD, FADH - reduced form: NADH, FADH2
|
|
|
ATP is generated by ______________and ______________ |
substrate-level phosphorylation and oxidative phosphorylation |
|
|
substrate-level phosphorylation |
a way of generating ATP in which a phosphate group is transferred to ADP from an organic molecule, which acts as a phosphate donor or substrate - produces only a small amount go the total ATP generated in the process pf cellular respiration |
|
|
oxidative phosphorylation |
- produces most of the ATP - ATP is generated indirectly in the reactions of 1- oxidative phosphorylation, through the reduction of electron carriers, 2- the transfer of high-energy electron transport carriers to the electron transport chain 3- subsequence synthesis of ATP from ADP and inorganic phosphate |
|
|
energy is released _______ in cellular respiration |
gradually - glucose is oxidized slowly through many reactions, and many of these reactions release energy in the form of ATP and electron carriers |
|
|
what is the starting molecule for glycolysis? |
glucose |
|
|
what does glycolysis mean? literally? |
sugar splitting - this is because glucose is split in two, yielding 3 carbon molecules - this process is anaerobic, because 02 is not consumed |
|
|
what are the steps in the three phases of glycolysis? |
1- frist ATP-consuming reaction ( PHASE ONE) 2- second ATP-comsing reaction ( PHASE ONE) 3- cleavage of 6-carbon sugar to two 3-carbon molecules ( PHASE 2, CLEAVAGE ) 4- PHASE 3 STARTS - NADH producing reaction - frist ATP producing reaction - second ATP-producing reaction IN THE END IT PRODUCES 4 ATP AND 2 NADH
|
|
|
total ATP yield for glycolysis |
substrate level - 2 ATP oxidative phosphorylation - 2 NADH = 5 ATP TOTAL = 7
|
|
|
total ATP yield for acetyl-CoA synthesis ( 2 pyruvate ---> 2 acetyl-CoA) |
substrate = 0 oxidation = 2 NADH = 5 ATP TOTAL= 5 |
|
|
citric acid cycle ( 2 turns, 1 for each acetyl-CoA) |
substrate = 2 ATP oxidative = 6 NADH= 15 ATP 2 FADH2 = 3 ATP total 20 |
|
|
the phosphorylation has two important consequences, what are they? |
- glucose can enter and exit the cells through specific membrane transporters, however phosphorylated glucose is trapped inside the cell. - the presence of two negatively charged phosphate groups in proximity destabilizes the molecule so that it can be broken down in the cell |
|
|
rhw oxidation of __________ connects glycolysis to the citric acid cycle |
- pyruvate - which is the end product of glycolysis
|
|
|
pyruvate |
- end product of glycolysis - contains a good deal of chemical potential energy can be further broken down to release more energy - first to acetyl-CoA and then in a series of reactions known as the citric cycle |
|
|
the mitochondrial membranes and compartments, what are they? |
- the space between the inner and outer membranes is the inter membrane space - the space inside the inner membrane is the mitochondrial matrix |
|
|
where does the formation of acetyl-CoA ( 1st substrate in the citric acid cycle), how? |
in the mitochondrial matrix - the synthesis of one molecule of acetyl-CoA from pyruvate results in the formation of one molecule of carbon dioxide and on molecule of NADH
|
|
|
pyruvate dehydrogenase complex |
a group of enzymes that catalyze the pyruvate reactions |
|
|
where where does the citric cycle take place? |
mitochondrial matrix |
|
|
what does the citric cycle produce? what is it? |
the acetyl group of acetyl-CoA is completely oxidized, with the net production of one ATP, three NADH, and one FADH2 - composed of 8 reactions and is called a cycle because the starting and ending molecule is oxaloacetate - produces a large amount of electron carriers ( donate high-energy electrons in the next stage of cellular respiration to power the synthesis pf ATP) |
|
|
can some bacteria run the citric acid cycle in reverse? |
YES running this cycle in reverse requires energy, which bacteria gets from sunlight/chemical reactions why? the intermediates generated step by step as the cycle turns provide the building blocks for synthesizing the cells biomolecules |
|
|
what does the citric cycle moving forward do for the cell? |
used to generate both energy-storing and intermediates in the synthesis of other molecules |
|
|
the electron transport chain transfers ______ and pumps _______ protons |
- electrons - protons |
|
|
coenzyme Q |
- transports electrons between the flour complex's, by accepting electrons form both complex 1 and 2 |
|
|
what are the two subunits that compose the ATP synthase |
F1 - uses the rotational energy made by the Fo to catalyze the synthesis of ATP Fo- forms a channel that rotates as protons pass through it |
|
|
when passing through complex's, what happens to the electons? what is the finial electron acceptor? |
the elections pass through electron donor and electron acceptors -each is a redox couple, consisting of a reduced and oxidized form of the molecules when passes through 1- they are reduced in electrons 2- O2 is the final electron accepter and has the highest electron affinity |
|
|
the _______ ________ is a source of potential energy |
proton gradient |
|
|
what is the proton gradient? what are it's components? |
- a difference in proton concentration across the inner membrane - often referred to as the electrochemical gradient - two components: = a chemical and electrical gradient |
|
|
chemical gradient |
difference in concentration |
|
|
electrical gradient |
the difference in charge between the two sides of the membrane |
|
|
how does the protein gradient store its energy? where is it's high and low concentrations? what does this cause? |
- store energy like a dam - high concentrations in the inter membrane space and a low concentration in the mitochondrial matrix = a tendency for protons to diffuse back to the mitochondrial matrix, driven by the difference in concentration |
|
|
what was mitchell;s hyothesis about how energy in stored in the cell? |
- 1961 - the gradient of protons provides a source of potential energy that is converted into chemical energy stored in ATP. for this energy to be released, their must be an opening for the protons to flow through.
- he thinks that protons in the intermedbrane diffuse down their electrical and concentration gradients through a transmembrane proton channel |
|
|
ATP synthase |
an enzyme that couples the movement of protons through the enzyme with the synthesis of ATP - forms the channel to the inner mitochondrial membrane through protons flow - made up of F0 and f1 |
|
|
how many molecules are produced of ATP for each NADH that donates electrons to the chain? ATP for each FADH2? |
- 2.5 molecules of ATP fro m NADH - 1.5 molecules of ATP from FADH2 |
|
|
fermentation extracts energy from _______ in the absence of ________? |
- glucose - oxygen |
|
|
fermentation |
a process of breaking down pyruvate through a wide variety of metabolic pathways that extract energy form full molecules such as glucose;
- does not relay on O2 or a similar outside electron acceptor |
|
|
lactic acid fermentation |
- animals and bacteria - electrons form NADH are transferred to pyruvate to produce lactic acid and NAD+ ( used in glycolysis) - this is the acid that builds up in your muscles when you work out, because e your muscles are not getting enough O2 |
|
|
ethanol fermentation |
- plants and fungi - pyruvate releases carbon dioxide to form acteldehyde, and the electrons from NADH are transferred to acetaldehyde to produce ethanol and NAD+ ( used in glycolysis ) |
|
|
why do NADH and NAD+ not show up for the chemical reactions involved with both ethanol and lactic fermentation |
because their is no net production or loss of either molecule |
|
|
explain the possible evolution of electron transport chain and oxidative phosphorylation |
- early cells envolved mechanisms to pump protons out of the cell, powered by ATP and electrons transport - eventually, electron-transport powered pumps became efficient enough to run the ATP-driven pump in reverse |
|
|
excess glucose is stored as ______ in animals and ________ in plants |
glycogen - animals starch - plants
|
|
|
the glucose concentration in your blood is highly controlled, how? |
high - glucose molecules that are not consumed by glycolysis are linked together to form glycogen in the liver and muscle
low glucose molecules located at the end of glycogen chains can be cleaved one by one, and are released, this will produce 3 not 2 molecules of ATP since the 1st of glycolysis is not done |
|
|
glycogen stored in the muscle, and in the liver, compare |
muscle = provide ATP for muscle contraction liver= central glycogen storehouse for the whole body, does not use it for itself ( release into blood stream when needed) |
|
|
what are the two types of sugars that contribute to glycolysis? what do they do? |
- disaccharides(two sugars) = maltose, lactose, sucrose, these are hydrolyzed into monosaccharides before being used
- monosaccharides ( one sugars) = fructose, mannose, galactose they become the intermediates of glycolysis, and they go into glycolysis further down the pathway |
|
|
the hydrolysis of some disaccharides produces glucose molecules that indirectly enter glycolysis, T or F |
F, they enter directly |
|
|
______ ______ and ________ are useful sources of energy |
fatty acids and proteins |
|
|
calories |
units of energy |
|
|
beta oxidation |
- does not produce ATP - releases however large amounts of NADH and FADH2 - the process of shorting fatty acids by a series of reactions that sequentially remove 2 carbon units form their ends |
|
|
what do NADH and FADH2 do? |
molecules that provide high-energy electrons for the synthesis of ATP by oxidative phosphorylation
|
|
|
the oxidation of fatty acids produces? |
- a large amount of ATP |
|
|
the intercellular level of _ _ _ is a key regulator of cellular respiration, why? |
ATP - holds energy in its bonds energy that can be used for all kinds of cellular processes |
|
|
ATP is being constantly broken down to what? what is it an indictor of? |
- being turned over in the cell, and broken down into ADP and Pi to supply the cell;s energy needs, and re-synthesized by fermentation and cellular respiration - this is the reason that it is an indictor how much energy is in the cell |
|
|
what happens when ATP levels are high in a cell? low? |
high amount of energy - pathways that generate ATP are slowed
low amount of energy - cell activates pathways to produce ATP synthesis |
|
|
high levels of NADH do what to cellular respiration |
inhibit cellular respiration |
|
|
high levels of NAD+ do what to cellular respiration |
stimulates cellular respiration |
|
|
what is an example of integrated metabolic control |
the regulation of PFK-1 ( glycolytic enzyme) - when ATP levels are low, it is activated, allowing glycolysis to continue - when ATP or citrate levels are high, it is inhibited and glycolysis slows |
|
|
what are the allosteric activators of PFK-1 |
ADP and AMP - they are abundant, one or the other will bind to the enzyme and cause the enzyme shape to change, the shape change activates the enzyme, increasing the right of glycolysis - to lower the production of ATP, it binds to the enzyme, and instead inhibits the enzymes catalytic activity |
|
|
what downstream product regulates PEK-1? |
citrate ( intermediate in the citric cycle) acts as an allosteric inhibitor of the enzyme, slowing its activity |
|
|
why can't runners maintain the pace of a sprint for longer runs? |
- muscle cells have a large amount of mitochondria, which produce ATP by aerobic respiration, the energy produced is much greater then fermentation, however is much slower, this is the reason |
|
|
water is a _________ __________ reaction |
oxidation reduction |
|
|
cells communicate using ___________ ________ that bind to specific receptors |
chemical receptors |
|
|
signeling cell |
source of the signalling molecule, which bonds to a receptor molecule on or in the responding cell |
|
|
pneumococcus, what is it? what does it do? |
- a disease causing bacterium - associated with pneumonia, meningitis and some kinds of arthritis - able to take up DNA from the environment and incorporate it into their own genome, this allows them to resist antibiotic drugs |
|
|
what allows bacteria such as pneumococcus to be able to communicate? |
- the rate of DNA uptake increases in large density of bacteria, this is because the concentration of the signalling peptide is high enough to stimulate the DNA uptake response, and in low concentrations they are unable to do this. |
|
|
signalling molecule |
- the carrier of information transmitted when the signalling molecule binds to a receptor - also can be called a ligand - |
|
|
receptor molecule |
the molecule on the responding cell that binds to the signalling molecule |
|
|
responding cell |
the cell that receives information from the signalling molecule |
|
|
signalling involves ________ _________, ________ ________, _________, and ___________ |
receptor activation, signal transduction, response, and termination |
|
|
what happens after a signalling molecule binds to a receptor on a responding cell? |
the receptor is turn on ( whoo), and then transmits the message through the cytoplasm, often by intracellular signalling |
|
|
signal transduction |
the signal is transmitted to the interior of the cell by a signal transduction pathway - carried through the cytosol or uncles, and along the way the initial signal is often amplified, so that the small signal can have a large affect on the responding cell - a step in cell signalling |
|
|
response ( cell signalling ) |
- can take different forms depending on the nature of the signal and the type of responding cell - the cell will respond, for ex: activating an enzyme or turning an transcription of a gene |
|
|
termination ( cell signalling) |
- allows the cel to respond to new signals - |
|
|
_________ signalling acts over long distances |
endocrine |
|
|
endocrine signalling |
signalling by means of molecules that travel through the bloodstream |
|
|
how are the types of signalling classified by? |
according to the distance between the signalling and responding cells |
|
|
what are some examples of endocrine-signalling molecules? |
- mammalian steroid hormones estradiol - testosterone |
|
|
_______ and _______ signalling act over short distances |
paracrine and autocrine |
|
|
paracrine signalling |
- involves two cells that are close together - the signalling molecule needs to diffuse only a short distance to the nearest neighbouring cell in order to bin it receptor and deliver its message - travels up to 20 cell diameters |
|
|
autocrine signalling |
- where the signalling cell and responding cell are one and the same - important during the development of the embryo - signalling cells are secreted by the cell and the bind to the receptors on the very same cell |
|
|
growth factor |
any one of a group of small, soluble molecules, usually the signal in paracrine signalling, that affect cell growth, cell division, and changes in gene expression |
|
|
where do growth factors come form |
- Kohler and lipton - that the growth promoting factor was activated or introduced into the blood by platelets during the clotting reaction |
|
|
what was the 1st growth factor named? |
platelet-derived growth factor |
|
|
how do growth factors work in the embryo |
- influence the kinds of cells their neighbours will become, they help shape the structure of the adults tissues, organs, and limbs.
|
|
|
what does the growth factor the sonic hedgehog do? ( NAMED AFTER THE DAM CHARACTER ) |
ensures that the motor neutrons in your spinal cord are located properly, the bones of you vertebral column form correctly and that your thumb and your picky fingers are on the correct sides of your hands |
|
|
juxtacrine signalling depending on direct ____ -______ contract |
cell-cell |
|
|
juxtacrine signalling |
- contact-dependent - without a chemical signal that diffuses or circulates through an external medium. - a transmembrane protein on the surface of one cell acts as the signalling molecules and a transmembrane protein on the surface of an adjacent cell acts as the receptor
|
|
|
in which from are electrons transferred during typical redox reactions such as the oxidation of glucose |
hydrogen atoms |
|
|
in which of the following molecules are the electrons of the storms shared in covalent bonds at the furthest distance form the nucleus? 1- water 2- glucose 3- carbon dioxide |
glucose |
|
|
PFK-1 is ___________ by ATP and _______ by ADP |
inhibited and activated |
|
|
what happens to pyruvate during the process of fermentation? |
it is reduced to ethanol |
|
|
in eukaryotic cells, the oxidation of pyruvate occurs in? |
the matrix of the mitochondria |
|
|
in the electron transport chain, what would have the lowest amount of energy? |
- the electron bound to oxygen ( in the form of water) |
|
|
which organisms would you expect to have ethanol fermentation |
fungi and plants |
|
|
when a single pyruvate is converted to acetyl-CoA, the other products of the reaction are? |
NADH and CO2 |
|
|
a researcher is studying a population of bacteria that carry out the critic acid cycle, but do so in reverse, what are you say for sure about the bacteria? |
will produce sugars from intermediates of the critic acid cycle |
|
|
during aptly-CoA synthesis, pyruvate is? |
oxidized |
|
|
fermentation takes place where? |
in the cytoplasm |
|
|
ligand-binding site |
the signalling molecule binds to a specific location on the receptor protein - causes the conformational change in the receptor, this activates the receptor, by passes th message from the dognaping molecule to the interior of the cell. |
|
|
true or false, the receptors can be o the cell surface or in the interior of the ell |
true |
|
|
what does the location of a receptor of a cell depend largely on? |
if the cell is polar or non-polar - |
|
|
cell-surface receptors |
- interact with polar signalling molecules that cannot cross the plasma membrane - polar molecules cannot cross the plasma membrane and rely on cell-surface receptors |
|
|
intercellular receptors |
interact with non polar signalling molecules that can cross the plasma membrane - small non-polor signalling molecules can freely pass through the plasma membrane and activate cytoplasmic receptors |
|
|
G protein-coupled receptor |
a receptor that couples to G proteins, which bind to the quanine nucleotides GTP and GDP |
|
|
G protons |
proteins that bind to GTP and GDP - when it is bound to GTP it is active, when it is bound to GDPit is inactive |
|
|
receptor kinase |
- second kind of receptor - enzyme that adds a phosphate group to another molecule in a process called phosphorylation |
|
|
phosphatases |
have the opposite effect and remove a phosphate group, a process dephosphorylation |
|
|
when a protein is phosphorylated by a kinase, it becomes ______ and when it is dephosphorylated by a phosphatase it is__________. |
- active, turned on - inactive, turned off |
|
|
ligand-gated ion channels |
- 3rd receptor group - alter the flow of ions across the plasma membrane when bound by their ligand - signalling molecule binds to the extracellular ligand-binding site of the channel protein, the channel undergoes a conformational change that opens it and allows ions to flow in and out - important for neurons and muscle cells - since primary function depends pm a rapid change in ion transport across the plasma membrane |
|
|
how do the delta and glial cells work? |
the delta is the signalling cell such as the neuron |
|
|
notch |
another world for the a glial cell, which is the supporting cell |
|
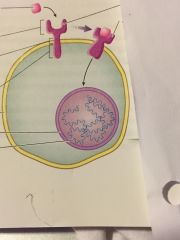
what type of receptor is it? name the components and explain whats going on |
- polar signalling molecules cannot cross plasma membrane and rely on cell-surface receptors - cell-surface receptor
the pink square = polar signalling molecule the fork like thing = extracellular domain components of it--> 1st part transmembrane domain and the cytoplasmic domain
the activated receptor will be swollen |
|
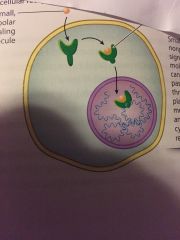
what type of receptor is it? name the components and explain whats going on |
- intracellular receptor - the orange ball = small, non polar signalling molecule --> can freely pass through the plasma membrane and activate cytoplasmic receptors
the activated receptor will be shorter, ( not as long as tail ), and then travel into the nucleus and into the DNA |
|
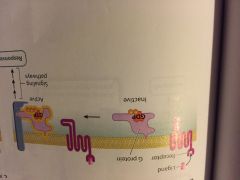
what type of cell-surface receptor is this? ( flip the picture) |
- G protein-coupled receptor |
|
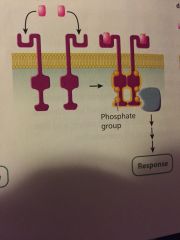
what type of cell-surface receptor is this? |
receptor kinase |
|
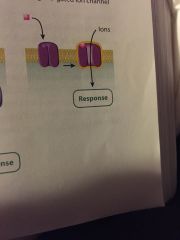
what type of cell-surface receptor is this? |
ligand-gated ion channal |
|
|
binding affinity |
the amount of time a signalling molecule remains bound to its receptor bound to its receptor depends on how tightly the receptor holds on to it |
|
|
amplification of G protein signalling, steps of G protein-coupled receptors |
- each activated receptor activities multiple G proteins which in turn activate adenylyl cyclase enzymes
- each adenylyl cyclase enzyme produces large amounts of the second messenger cAMP, which activates many molecules of proteins kinase A
- each protein kinase A enzyme phosphorylates and activates multiple protein targets |
|
|
termination of a G protein signal - 4 steps |
- termination at several places, allowing the cell to respond to new signals 1- phosphatase remove phosphate groups from proteins, causing them to become inactive 2- the signal molecule adrenaline detaches from the receptor after a certain amount go time, inactivating the receptor so that it can no longer bind to and activate the G protein 3- enzymes in the cytosol specifically degrade cAMP which stops the phosphorylation and activation of target proteins by PKA 4- within a very short time, an activated G protein deactivates itself by converting GTP to GDP |
|
|
receptor kinases _____________ each other and activate intracellular signalling pathways |
phosphorylate |
|
|
does the receptor kinase signalling in embryonic development do? |
- the formation and elongation of structures called limb buds that eventually become our arms and legs |
|
|
what happens when you cut your finger? which enzyme etc? |
- platelet-derived growth factor is released from platelets in the blood and binds to its receptor kinase on the surface of the cells at the site of the wound - it triggers the cell division necessary to repair the damaged tissue |
|
|
what is kit? |
- receptor kinase - important for the production of pigment in skin, feathers, scales, and hair |
|
|
what does the receptor kinase activation and signalling work? |
- receptor kinases bind signalling molecules, dimerize, phosphorylate each other, and activate intracellular signal molecules |
|
|
dimerization |
- activates the cytoplasmic kinase domains of that paired receptors, causing them to phosphate to each other at multiple sites on their cytoplasmic tails - when a signalling molecule binds to the extracellular portion of the receptor, a conformational change cases the receptor to partner up with another receptor kinase bound to another molecule of the same ligand |
|
|
______ has a lot to do with getting the healing process stated? |
PDGF |
|
|
Ras |
cytoplasmic signalling proteins - in the absence of a signal, Ras is bound to GDP and is inactive - Ras binds to an activated receptor kinase, it releases GTP and binds to GTP to become active - activated GTP-bound as tigers the activation of a series of kinases that together are called the mitogen-activated protein (MAP) kinase pathway |
|
|
the final activated kinase in the series enters what? |
- nucleus, where it phosphorylates it target proteins - some of these proteins = regulators of transcription that switch on genes needed for the cell division so that your cuts and such can heal |
|
|
how is the receptor kinase signalling is reversed or terminated by what basic mechanisms? |
- G protein-coupled receptor pathways |
|
|
ligand-gated ion channels |
- ion channels to regatta the permeability of the cell membrane to ions - |
|
|
what contributes to a difference in electrical charge across the plasma membrane? |
the difference in NA+ concentration |
|
|
membrane potenial |
the difference in electrical charge across the plasma membrane
|
|
|
at rest, the inside of the cell has a ________ charge, what is this a result of? |
negative charge relative to the outside - the large amount of positively charged sodium ions outside the cell and the high concentration of negatively charged ions and proteins in the cytosol |
|
|
when the channel is opened through ligand-gated ion channels, what happends? |
- allows NA+ to move down its concentration gradient into the cell - causes a charge difference between the outside and inside of the cell. - the neutron responds by sending a nerve impulse and the muscle cel responds by contracting
|
|
|
what does acetylcholine binding do? |
- opens ion channels allowing NA+ to flow into the muscle cell - ligand-gated ion channal |
|
|
which type if receptor is involved in rapid response of muscle cells and nervous? |
ligand-gated ion channal
|
|
|
a protein on a cell surface that binds to a sigalling molecule is an example of which of the following elements of cellular communication 1- a receptor molecule 2- a signalling molecule 3- none 4- a responding cell 5- a signaling cell |
a receptor molecule |
|
|
A G protein does what exactly? |
1- becomes deactivated when bound GTP is dephisphylated to GDR 2- composed of a signal-transduction pathway that is coupled to a G protein coupled receptor 3- is composed of three subunits and is inactive when bound to GDP 4- releases GDP and bonds to GTP when associated with an activated receptor |
|
|
what processes are part of the communication of a neuromuscular auction? |
- a open ligand-gated ion channel allows NA+ to rush across the plasma membrane - a cell-surface receptor on the muscle cell us activated by acetylcholine binding - a neuron releases a neurotransmitter to signal contraction by the muscle cell - a sudden influx of NA+ across the plasma membrane drastically reduces the membrane potential and activates the contractile memory of the muscle cell |
|
|
why do the functions of many receptor kinases depend on the fluid nature of the plasma membrane? |
- the receptor monomers must move together and depends to be activated |
|
|
the delta protein does what? |
- directs adjacent cells to differentiate into glial cells - it is transmembrane protein found in adjacent cells - arises in embryonic stem cell as they differentiate into neurons in the brain |
|
|
kohler and lipton first discovered a growth factor (PDGF) by? |
- observing that fibroblasts grew better in cell culture when blood sure was added to the growth medium instead of blood plasma |
|
|
platelet-derived growth factor (PDGF) is a signalling molecule that functions in which of the following types of signalling? |
paracrine |
|
|
the MAP kinase pathway, what happens exactly? |
- some receptor kinases signal through RAS, which in turn activates the MAP kinase pathways, leading to changes in gene expression 1- a small amount of signal received by receptor kinase is amplified when the signal is passed from kinase to kinase as each is phosphorylated
|
|
|
true or false, no more then one signalling pathway can take place simultaneously in a signal cell |
- false, the cellular response depends on the integration of several signals |
|
|
mutations in the receptor kinase and RAs are frequently associated with _________? |
human cancer |

