![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
Metabolism
|
a series of chemical reactions
two pathways: anabolic and catabolic |
|
|
Anabolic Metabolism
|
building, requires energy
|
|
|
Catabolic Metabolism
|
break down, releases energy
|
|
|
Order of metabolism
|
1. catabolic, 2. anabolic
|
|
|
Energy
|
the ability to do work
|
|
|
Potential Energy
|
energy based on location and structure, chemical
|
|
|
Kinetic Energy
|
energy of motion, heat
|
|
|
Thermodynamics
|
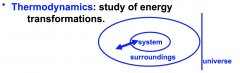
study of energy transformation
|
|
|
System
|
where the things are happening
|
|
|
Surroundings
|
around what's happening
|
|
|
Universe
|
both system and surroundings
|
|
|
Open system
|
moving of energy (a cell)
|
|
|
Closed system
|
no movement of energy
|
|
|
1st Law of Thermodynamics
|
energy cannot be created, but transformed
|
|
|
2nd Law of Thermodynamics
|
disorder of universe increases, energy lost by heat
|
|
|
_____ must conform to both 1st and 2nd Laws of Thermodynamics
|
cells
|
|
|
Free Energy
|
energy of a system that can do work (at uniform system temperature)
Ex: cells |
|
|
Free Energy Chemical Reaction
|
G = H-TS
If products less than reactant, G is negative If products more than reactants G is positive |
|
|
Exergonic
|
more stable, spontaneous, ball down hill motion
NEGATIVE G |
|
|
Endergonic
|
less favorable, ball up hill motion, non spontaneous
POSITIVE G |

