![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
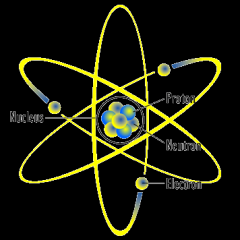
Atom: The smallest unit of an element that maintains the chemical properties of that element |
|
|
|
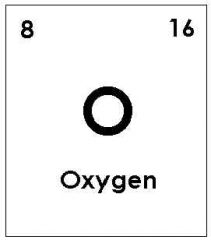
Element: A substance that cannot be separated or broken down into simpler substances by chemical means; all atoms of an element have the same atomic number. |
|
|
|
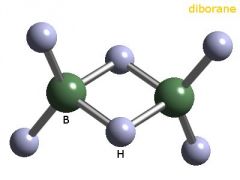
Compound: A substance made up of atoms of two or more different elements joined by chemical bonds. |
|
|
|
Physical Property: A characteristic of a substance that does not involve a chemical change, such as density, color, or hardness. |

|
|
|
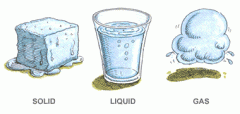
Physical Change: A change of matter from one form to another without a change in chemical properties. |
|
|
|
Chemical Property: A property of matter that describes a substance's ability to participate in chemical reactions. |

|
|
|
Chemical Change: A change that occurs when one or more substances change into entirely new substances with different properties. |

|
|
|
Homogeneous Mixture: A mixture that has uniform structure or composition throughout. |

|
|
|
Heterogeneous Mixture: A mixture that is not uniform in composition – it's a non-uniform mixture of smaller constituent parts. |

|
|
|
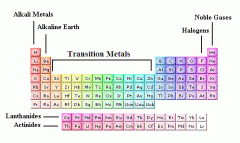
Group: A vertical column of elements in the periodic table; elements in a group share chemical properties. |
|
|
|
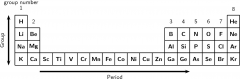
Period: In chemistry, a horizontal row of elements in the periodic table. |
|
|
|
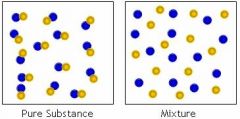
Pure Substance: A sample of matter, either a single element or a single compound, that has definite chemical and physical properties. |
|

