![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
|
What determines gram pos vs gram neg?
|
peptidoglycan
gram negs also have an outer membrane with lipopolysaccharide |
|
|
What allows certain bugs to be stress-resistant in the dormant phase of bacterial development?
|
Bacterial endospores.
Little bag of DNA covered by peptidoglycan |
|
|
What is a virulence factor?
What are the general categories that virulence factors can target? (5) |
bacterial products, or strategies, used to contribute to the ability of an organism to perpetuate itself and/or cause disease.
Allow attachment and/or invasion Allow acquisition of crucial nutrients Inhibit phagocytic processed evade immune responses Directly damage the host In many cases a pathogen will have a spectrum of virulence factors- many tools are used to satisfy the four steps of survival. |
|
|
What are three ways that pathogens can release virulence factors?
What is the goal of virulence factors? |
Virulence factors can be _surface localized__ or __secreted_ by the pathogen- less common: cytoplasmic or inner membrane bacterial proteins- but there are examples of such proteins that are absolute determinants of virulence.
Examples: Shigella and acid resistance. Regulatory proteins expressed only during infection The goal: overcome any barriers to using the host as a growth environment. |
|
|
What are the physiological barriers to infection? (3 listed here)
|
Skin- essentially no bacteria can penetrate intact skin.
barriers to infection include: lack of moisture- microbes need moisture to grow. acid environment- free fatty acids produced by normal skin flora and secreted by sebaceous glands are inhibitory to most pathogens. |
|
|
Define adhesins
|
microbial surface components that bind to a host cell receptor. They are factors that assist in attachment and colonization.
|
|
|
What are the proteinaceous adhesins of bacteria and what is their structure?
|
Fimbriae (adhesive pili): long structures that extend off the surface of a bacterium. Mediate attachment.
Fimbriae structure: Composed mostly of _pilin_- a protein arranged in an extended homopolymeric helical structure. There are in many cases unique proteins that are localized to the tip of the individual fimbriae. In other cases the pilin protein is responsible for the binding. |
|
|
What part of the proteinaceous adhesins of bacteria mediates attachment?
|
The tip of the fimbriae mediates attachment. This is generally quite specific, with a certain receptor bound by a specific fimbriae.
|
|
|
What do the proteinaceous adhesins of bacteria bind to on the host cell surfact?
|
Fimbriae bind to __carbohydrate__ residues of glycolipids or glycoproteins at the host cell surface. Binding by fimbriae is commonly an __initial__ point of contact.
|
|
|
What aspect of pili may assist in immunologic avoidance of bacteria?
|
Microorganisms are constantly producing and _shedding_ pili- may assist in immunologic avoidance.
|
|
|
What are afimbrial adhesins?
What type does streptococcus use? What about Yersinia? Chlamydia? |
Afimbrial adhesins- surface proteins not structurally related to fimbriae. Not structurally organized like pili.
Much variation in structure and function. Streptococcus- F protein- adherence to __fibronectin. Yersinia invasin- binds to host cell surface protein (integrin); mediates invasion. Chlamydia MOMP proteins- adhere to heparin- a glycosaminoglycan. |
|
|
What do afimbrial adhesins bind to?
|
Binds to proteins or carbohydrates- often forms tight associations. Often a host cell serum factor is an intermediate in the binding. Can be a dominant protein in the bacterial cell.
|
|
|
What are the non-proteinaceous adhesins of bacteria?
|
lipoteichoic acid - lipid-linked teichoic acid-
found in gram positive cell wall. teichoic acids are complex polymers consisting of glycerol, a sugar (ribitol), and phosphate linkers. ! Can be 50% of all cell wall material in G+ bact.! capsular polysaccharide - Capsules are collections of long chain _polymers__ of different simple components-! Exopolypeptide capsule- Bacillus, consists of repeating D-glutamic acid.! __exopolysaccharide_ capsule- many organisms, many different carbohydrates. ! |
|
|
What are the functions of the capsule?
How do they play into immune avoidance? |
Functions of capsule: __adherence, immune avoidance_
Regarding immune avoidance: capsules function in three ways- _antiphagocytic___ antigenic mimicry antigenic ___masking__ |
|
|
Why are biofilms so difficult to treat?
|
Get these notes from someone.
free-living bacteria that make up biofilms change completely when they are making up the biofilm. This is key because it effects how we treat them. The free-living ones grown on petri dishes tend to be sensitive to a lot, but as a biofilm is incredibly resistant to the same antibiotics it was once susceptible to. The biofilm forms a phsyical barrier for both antibiotics and immune mediated response cells. |
|
|
Why do biofilms cause such a problem in recurring infection?
|

It doesn't take many for them to get going, and once strong, they send out streamers to colonize new places.
|
|
|
what are some examples of surface factors on bacteria?
|
attachment, invasion, immune avoidance, virulence factors.
|
|
|
What are host iron chelators?
|
transferrin, lactoferrin
|
|
|
One nonspecific humoral host defense mechanism is a plasma protein that activates complement, what is this protein?
|
Properdin
|
|
|
What innate immunity of the host is a protein with activity against gram positive cell wells?
|
beta-lysin
|
|
|
What are acute-phase proteins?
|
Part of the innate host immunity, acute phase proteins are plasma proteins that elevate following infection/trauma. C-reactive protein- an acute phase protein that activates the complement pathway.
|
|
|
What are the bacterial iron grabbers? How do they do this?
What are other bacterial iron sequestering mechanisms? How do these grabbers play into the fight against bacteria? |
Siderophores - secreted metabolites used by many species to compete for iron. Siderophores have very high affinity for iron. These molecules “steal” iron from transferrin, lactoferrin.
In some cases production of siderophores are absolutely required for virulence. Other iron sequestering mechanisms: Cell surface proteins that bind hemin, transferrin, etc. for scavenging iron. hemolysins that free iron binding proteins within red blood cells. Common tack to outwit bacteria - undo their siderophores. |
|
|
What stimulates the complement pathway?
|
Microbial products or via specific antibodies.
|
|
|
What are the two arms of the complement pathway?
What is its primary goal? |
It has 2 arms- one is used in the innate immune mechanism (the alternate pathway), one is antibody dependent and is used in the acquired immune system (the classical pathway).
Its primary goal is to generate the___membrane attack complex_____- this is a collection of several complement proteins that form a stable pore in a target membrane and disrupt the ionic balance within the cell. |
|
|
What does activation of the complement cascade produce and what is the function of the products?
|
Activation of the complement cascade produces peptide products that are ___chemoattractants____ and stimulants for other immune mechanisms.
|
|
|
How do capsules interact with complement?
|
capsules block complement.
|
|
|
How do pathogens combat the complement pathway? (two ways).
What happens to a pathogen that cannot combat complement? |
Some bacteria have unique surface structures that block complement activation. Many other surface factors inhibit complement activity. If these factors are missing from a pathogen, virulence is often reduced.
|
|
|
T/F Neutrophils are phagocytic.
Are neutrophils dividing cells? What level of responder are neutrophils? Where do they circulate? |
True
neutrophils- polymorphonuclear cells. PMN’s. very short-lived, nondividing cells. The first cells that attack invading organisms in a naive animal. Circulate in the bloodstream. |
|
|
T/F macrophages are phagocytic
How long do macrophages live? Where do they circulate? What is an inactivated macrophage in the bloodstream called? |
True
macrophage, mononuclear cells, long lived cells that take many forms within the body. These cells are “stationed” at various spots in the body (lung, liver, spleen, brain, etc.) as well as circulating in the bloodstream. Inactivated macrophage in the bloodstream are called monocytes. |
|
|
What signals are phagocytic cells attracted to?
|
Phagocytic cells are attracted to an area via signals sent by damaged cells or by invading organisms. Signals include:__complement_ products, clotting products, bacterial __LPS_, formyl peptides.
|
|
|
What are the steps of ingesting and killing microbes by a neutrophil?
|
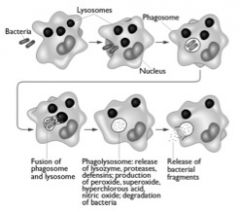
Binding of opsonins triggers phagocytosis-
(eating cells)- ! The target is endocytosed- a vacuole is formed! Toxic products are dumped into the vacuole! The target is killed and processed for exocytosis or for antigen processing.! The vacuole- known as a _______________- is a! very hostile environment. ! Multiple killing mechanisms! oxygen dependent- “chemical warfare”! oxygen independent- “enzymatic warfare”! |
|
|
How are bacteria and parasites recognized by phagocytic cells?
|
Bacteria and parasites are recognized through binding by _opsonin__-plasma proteins that bind to microbial products.
Examples: LPS-binding protein C3b receptor Antibody- acquired immune response |
|
|
How do capsules effect phagocytosis?
|
With a capsule, the phagocytic cell just pushes the pathogen along unable to gain purchase to phagocytose it.
|
|
|
Many systemic pathogens produce extracellular
DNAse. Similar nonpathogenic species often do not. Why? |
Not sure, missed it. Ask someone or look it up.
Does it have something to do with: Extracellular killing by neutrophils. NETs- Neutrophil Extracellular Traps. NETs contain chromatin (DNA), histone, and enzymes. |
|
|
The bacteria that live and survive within organelles require how many rounds of colonization?
|
There are several unique sites within the cell that can allow growth of microorganisms. cytoplasm, endosomes, pre-lysosomes, Golgi or E.R-like vesicles, even full blown lysosomes!
These environments require the four steps of colonization to occur twice- within the organism and within the cell. |
|
|
Intracellular parasites do one of three things to handle getting phagocytosed. Name the three mechanisms and name two organisms that practice each.
|
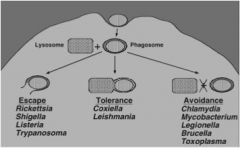
see the pic
fyi - intracellular parasites can be either facultative or obligate. also fyi - recent study has found that the lysosomal shrapnel created by the escape artists (Shigella) trigger the host to target the cell for destruction |
|
|
What mechanism do listeria use for motion?
|
Actin
|
|
|
What are the non-proteinaceous adhesins?
|
Lipoteichoic acid- lipid-linked teichoic acid- found in gram positive cell wall. teichoic acids are complex polymers consisting of glycerol, a sugar (ribitol), and phosphate linkers. Can be 50% of all cell wall material in G+ bact.!
|

