![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
What is a anaerobic pathogenic bacterium?
What protects the bacterium from oxygen toxicity? What are some virulence factors that aid in establishment of low oxygen microenvironment? |
Requires absence of oxygen for growth
Sensitive to oxygen reactive species Superoxide dismutase, catalase and NADH oxidase protect from oxygen toxicity Establishment of anaerobic microenvironment (low redox potential) or low oxygen tolerance is essential for infection. Endotoxins, hemolysins are some virulence factors that aid establishment of low oxygen microenvironment |
|
|
Compare and contrast gram + and gram - anaerobic bacterial pathogens?
Which one forms spores? Which group has more diversity? |
Gram-positive
!! Spore formers !! Clostridium genus !! Spores are the infectious morphotype !! Spore germination is essential for disease Gram-negative !! Non-spore formers !! Bacteroides genus !! Brachyspira genus !! Fusobacterium necrophorum !! Dichelobacter nodosus !! Porphyromonas genus !! Prevotella genus !! Vegetative cells are the infectious morphotype |
|
|
Can you kill gram + anaerobic pathogenic bacterium by heat or desiccation?
|
No, because of their protective spores.
Enriched media required for growth |
|
|
What are the three classifications of gram + anaerobic pathogenic bacterium?
|
Neurotoxic clostridia
!! Clostridium botulinum !! Clostridium tetani Histotoxic clostridia !! Clostridium perfringens !! Clostridium septicum !! Clostridium chauvoei !! Clostridium sordelli Enteric clostridia !! Clostridium perfringens !! Clostridium difficile !! Clostridium septicum |
|
|
What is the Clostridial life cycle?
|
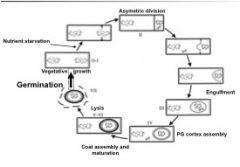
see above
|
|
|
What are the infectious morphotypes of clostridia?
|
The spores.
They are ubiquitously found in the environment and enter the host through the GI tract or open wounds. The presence of species-specific germinants trigger spore germination. The germinated spores release nascent cells, multiply and release toxins. |
|
|
What toxin does Clostridium botulinum contain?
|
Botulinum neurotoxin (BoNT), the most poisonous substance known.
BoNT can be produced by phenotypic and genetically different species of anaerobic gram-positive spore forming clostridia. Making Clostridium botulinum a taxonomic collection of different species. !! Some strains of Clostridium baratii and Clostridium butyricum can also produce BoNT. !! Physiological characteristics leads to four different groups (groups, I, II, III and IV). |
|
|
What does gene location of BoNT on the chromosome effect?
|
!!BoNT can be classified into seven serologically distinct groups,
based on the recognition by polyclonal serum. !! BoNT is encoded by approximately 3.8-kb gene that can be either chromosomally located or in a large plasmid. |
|
|
What are the seven types of BoNT?
|
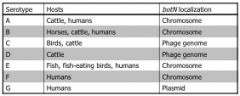
see pic
|
|
|
What is the epidemiology of the different strains of C. botulinum?
|
!! Botulism spores are ubiquitous in soils in most areas of the United States.
!! Spores of Type A are common in Mid west Pacific states. Not found in Mid-east Atlantic states. !! Spores of Type B are common in soil of mid-Atlantic. Responsible of 85% of botulism cases in cattle and horses. !! Type C and D associated with feed contaminated by decomposing carcass. Ravens feeding on a carcass transport BoNT to containers. !! Type D botulism is more common in South America and South Africa. !! Type E occurs in humans following consumption of fish. 20-50 human cases in Pacific Northwest annually. !! Type F and G not reported in domestic animals. |
|
|
What is the pathogenesis of C. botulinum? What are the 3 clinical forms?
How long does it take clinical symptoms to manifest? |
!! Germination of dormant spores is essential for BoNT production.
!! Three clinical forms of botulism: !! Food borne botulism: when food contaminated with the preformed BoNT is eaten. (i.e., contaminated feed). !! Intestinal botulism (toxico-infection): when C. botulinum grows in the gastrointestinal tract and BoNT is absorbed. !! Wound-related botulism: occurs when spores germinate in anaerobic abscess wound and produce toxin. !! Clinical symptoms manifests within 1 to 10 days depending on dosage. Include: progressive weakness due to flaccid paralysis of muscle, respiratory failure and death. |
|
|
What is the BoNT mode of action?
What does lack of SNARE complex impair? |
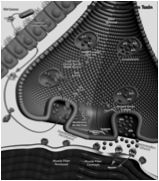
Toxin must reach general
circulation for its lethal effects to manifest. !! BoNT is actively transported through the gut lining, involving receptor-mediated transcytosis (vesicles employed for transport). Binding domain: recognizes specific receptors on neural cells. !! BoNT does not penetrate brain parenchyma !! Translocation domain: is involved in pore formation in the endocytotic vesicles to deliver the catalytic domain into the cytoplasm. !! Catalytic domain: is a zinc metalloprotease that cleaves a specific bond in one of the three neuronal SNARE proteins !! Each BoNT serotype cleaves a unique peptide bond in and different substrates. !! Lack of SNARE complexes impairs Acetylcholine release into the neuromuscular junctions. |
|
|
What are the three domains of C. botulinum?
|
binding domain
translocation domain catalytic domain |
|
|
What is the main treatment for C. botulinum?
|
!! Antitoxin must be administered before much toxin
has bound presynaptic nerve endings !! Mechanical ventilation !! Recovery takes weeks to months and occurs when new presynaptic end plates and neuromuscular junctions are regenerated. |
|
|
Is there immunity or vaccine to C. botulinum?
|
!! Animals would develop an antibody response to BoNT
3-4 weeks following recovery. !! Toxoid vaccine for C. botulinum type B is available, and three doses at 1-month intervals are required to successfully immunize horses. !! Toxoid: bacterial toxin (usually exotoxin) whose toxicity has been weakened or suppressed either by chemical (formalin) or heat treatment, while other properties, typically immunogenicity, are maintained. |
|
|
What type of pathogen is Clostridium tetani (some main characteristics)?
|
!! Is moderately fastidious anaerobe, and will grow on
agar media only under strong reducing conditions. !! Spores are ubiquitous in the environment, but more concentrated in some geographical areas than others. |
|
|
What is the pathogenesis of C. tetani?
What species is particularly susceptible to tetanus? How long can the spores lay dormant? What is the tetanus toxin virulence factor? |
!! Similar as Botulism: germination of dormant spores is
essential to return to vegetative growth and produce TeNT. !! Portals of spore entry are: contaminated traumatic wounds: castration band-induced lesion is sheep, goats and cattle; Tail-docking wounds and dehorning sites in adult cattle. !! Horses are particularly susceptible to tetanus, mainly contaminated wounds. !! Tetanus spores may remain dormant in healed wounds, with latent periods of up to 10 years. !! Tetanolysin: is an oxygen-labile hemolysin that inhibits chemotaxis of phagocytes and may also increase local tissue injury, enhancing bacterial replication and production of TeNT. |
|
|
What is the mode of action of C. tetani's virulence factor, TeNT?
|
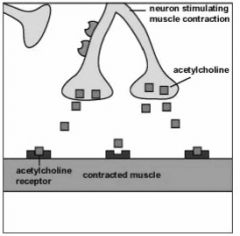
!! Mode of action of TeNT:
!! Has three domains: binding, translocation and catalytic domain. !! Binds to inter-neurons of the spinal cord. !! Blocks neurotransmitter release of inhibitors. !! The toxin, by blocking the release of inhibitors keeps the involved muscles in a state of contraction leading to spastic paralysis. |
|
|
What is the recommended treatment for C. tetani?
|
!! Antibiotics (metronidazole) to kill the bacteria,
tetanus booster shot, if necessary, and occasionally, antitoxin to neutralize the toxin !! Ventilator support to help with breathing in the event of spasms of the vocal cords or the respiratory muscles !! Rehydration because, as muscles spasm constantly, increased metabolic demands are placed on the body. |
|
|
What is the vaccine/immunity for C. tetani?
|
!! Immunization with tetanus toxoid provides
excellent protection for horses and humans. !! Annual revaccination of horses is recommended. |
|
|
What are the three common themes of infection of histotoxic clostridia?q
|
Common themes of infectious are:
!! Acquisition of spores of infectious organism from soil or intestinal tract. !! Entry to tissue following trauma. !! Local multiplication, extensive local and systemic tissue myonecrosis and rapid death. !! The hallmark is overwhelming toxinogenesis. !! Vaccination of animals decreases the incidence |
|
|
What is the major histotoxic clostridia and what does it do to the tissues?
|
!! Common species involved in tissue
myonecrosis: !! Clostridium septicum !! Clostridium sordelli !! Clostridium chauvoei !! Clostridium perfringens (Major) |
|
|
What are the two kinds of disease that C. perfringens can cause?
|
!! Can cause both histotoxic and enteric disease.
!! C. perfringens is the most important cause of clostridia disease in domestic animals. Can produce more than 17 toxins. |
|
|
What is the risk for C. perfingens having so many toxin types?
|
!! Other toxins are present in large plasmids and can be
transferred between strains through horizontal gene transfer (i.e., conjugation shown by several studies) |
|
|
Which type of C. perfringens are the main causative agent of myonecrosis?
|
C. perfringens type A isolates are the main causative
agent of myonecrosis. |
|
|
What are the virulence factors of C. perfringens?
|
Virulence factors:
Alpha toxin and Perfringolysin main toxins in the pathology of gas gangrene. Individual mutations in pfoA and cpa revealed synergistic effects of the two toxins in a mouse model. You need both to make a fully virulent infection. Individual mutants had slower spread of the infection and slower rate of death. Precise roles of each toxin are not clear. |
|
|
What is the vaccine/immunity of C. perfringens?
|
!! Immunization of domestic animals against
infection is not common in U.S.A. Due to lack of available vaccine Antibodies against CPA protect against mice challenged with toxin of C. perfringens. |
|
|
What type of C. perfringens is the most widespread cause of enteric disease?
|
C. perfringens is the main etiological agent of enteric diseases in animals causing great economical impact.
Potent toxins produced by C. perfringens are dominant attributes in the gut. Strains of type A are the most widespread toxinotype in the intestines of animals and in the environment. |
|
|
What are the chronic, acute, and global impacts of necrotic enteritis caused by C. perfringens?
|
!! C. perfringens is the main causative agent of avian NE.
!! NE manifest as an acute or chronic enterotoxemia. !! Acute diseases has significant mortality rate. !! Chronic disease leads to loss of productivity and welfare concerns. !! Disease costs the International Poultry Industry ~ US$ 2 Billion dollars annually. |
|
|
what are the virulence factors secreted by C. perfringens to cause necrotic enteritis?
|
Alpha toxin was believed to be the major virulence factor
cpa knock out mutant was still able to cause NE in chickens. So Alpha toxin does not cause the disease. They found another virulence factor toxin: Necrotic enteritis toxin B (NetB) Secreted to environment Belongs to the Leukocidin/Hemolysin family of toxins Forms hydrophilic pore in the cell membrane with a functional diameter of ~ 1.6-1.8 nm |
|
|
What are the current vaccines to C. perfringens?
|
To be used to prevent Necrotic enteritis.
Current vaccination uses alpha toxin toxoid provides partial protection. New vaccines based on NetB under development are promising. |
|
|
What does C. perfringens type A cause?
|
!! C. perfringens type A food poisoning
!! Ranks third most common food borne illness in humans in US. !! Also causes important enteric illness in animals !! Self limiting |
|
|
What is the pathogenesis of C. perfringens type A?
|
!! Pathogenesis
!! Consumption of contaminated food with C. perfringens type A enterotoxic-positive strains !! Vegetative cells initiate sporulation in the GI tract !! Clostridium perfringens enterotoxin (CPE) is released upon cell lyses of the sporulating cell !! Soft-watery diarrhea, sometimes with blood and mucus in dogs and pigs. |
|
|
What is the virulence factor of C. perfringens type A?
What is the mode of action in food poisoning? |
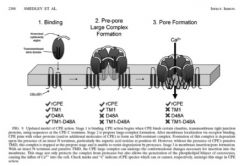
!! Clostridium perfringens enterotoxin (CPE)
!! ~ 35 kDa polypeptide !! Synthesized uniquely during sporulation !! Causes tissue damage, manifesting as diarrhea, cramps and fever. See pic for mode of action |
|
|
What immunity or vaccine is available again C. perfringens type A against food poisoning?
|
!! Although clinical disease give rise to serum
antibodies to CPE, they do not protect. !! No commercial vaccine available due to self- limiting and very low mortality rate. |
|
|
What clostridium strain is responsible for ~25% of all casses of antibiotic associated diarrhea?
|
Clostridium difficile
|
|
|
Is C. difficile infection zoonotic?
|
!! C. difficile infection is an emerging zoonosis (hyper-
virulent strains also found in feces of calves and meat products). !! C. difficile can be isolated from marine sediments, soil, feces of healthy humans and many domestic animals. !! Spores are the means of transmission of C. difficile, and can persist for many years. |
|
|
What is the pathogenesis of C. difficile associated disease?
|
Host exposed to antibiotic treatment suffers
reduction of normal gut flora. !! Clindamycin, ampicillin, amoxicillin, cephalosporin and other are associated with CDAD. !! Spores sense bile salts and germinate in the GI tract, vegetative cells multiply rapidly, filling empty niches and producing toxins. !! Toxin A and B are the major virulence factors. !! Disease may present as diarrhea, colitis, pseudo membranous colitis, or fulminant colitis. Gross pathology includes moderate to severe mesocolonic edema. !! Colonic serosal and mesenteric edema and infiltration of mononuclear inflammatory cells and neutrophils in the edamatous areas are common. !! Epithelia necrosis, ulceration and pseudo membrane formation has been observed. |
|
|
What are the virulence factors of C. difficile associated disease?
|
!! Toxin A (TcdA): monomeric enterotoxin, 308 kDa.
!! Toxin B (TcdB): monomeric cytotoxin, 270 kDa. !! TcdA and TcdB are glycosyltransferases !! Both belong to the structurally homologous family of large clostridial cytotoxins. !! Recently, TcdB has been identified as the sole virulence factor for CDAD (Lyras 2009 Nature). |
|
|
What are the modes of action of toxins A and B of C. difficile?
|
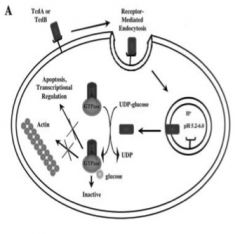
TcdA and B bind to non-protein receptor
!! Enter the cell via receptor-mediated endocytosis !! Decrease of liposome pH induces release of TcdA and TcdB !! TcdA & TcdB inactivate Rho, Rac and Cdc42 via transfer of sugar moiety, using UDP- glucose as a co-substrate !! Promoting actin condensation, transcriptional activation and apoptosis. |
|
|
What is the treatment of C. difficile?
|
!! Oral delivery of Metronidazole or Vancanomycin only
if metronidazole treatment fails. !! After antibiotic suppression high rate of relapse. Presumably, C. difficile spores germinate and re- colonize the GI tract before commensal flora Current challenging field of research |
|
|
Is there a vaccine available against C. difficile?
|
!! Antibodies against TcdA and TcdB prevent clinical
disease. !! ~ 60% of adults have antibodies against TcdA & TdcB, due to repeated intestinal exposure. !! Protective immune response only rises against repeating or relapsing infections. |

