![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
159 Cards in this Set
- Front
- Back
|
opioid definition
|
term used to describe any substance, natural or synthetic, which binds specifically to opioid receptors and produces some agonist (morphine-like) effects
|
|
|
what is unique about opioids?
|
they produce analgesia without loss of touch, proprioception, or consciousness
|
|
|
clinically used opioids and comparative potencies
|
Morphine 1
Meperidine 0.1 Fentanyl 75-125 Sufentanil 500-100 Remifentanil 250 |
|
|
what is the potency of opioids related to?
|
the sterochemical structure of the molecule
|
|
|
what structure is most active?
|
levoisomers (the left light-bending form of the enantiomers) (the naturally occuring form of morphine is the levoisomer (l-morphine)
|
|
|
semisynthetic opioids
|
result from the minor modification of the morphine molecule
|
|
|
synthetic opioids
|
contain the phenanthrene nucleus of morphine, but are made rather than just modifying the naturally occuring morphine
|
|
|
mechanism of action of opioids
|
act as agonists at stereospecific opioid receptors in the CNS and peripheral tissues, by mimicing endogenous peptide opioid receptor ligands (endorphins) by binding to the opioid receptors which activates pain-modulating (antinoceptive) systems
|
|
|
effect of opioid receptor activation
|
increased potassium conductance leading to hyperpolarization of neuron cellular membranes, causing a decrease in neurotransmission due mostly to inhibition of presynaptic neurotransmitter release (esp ACh and substance P)
|
|
|
what does depression of cholinergic transmission due to activation of opioid receptors lead to?
|
opioid-induced inhibition of ACh release at nerve terminals results in significant analgesic properties, and the other side effects of opioids, such as respiratory depression
|
|
|
types of opioid receptors
|
classified as mu, delta, or kappa
|
|
|
mu receptors
|
morphine-preferring receptors, which are principally responsible for supraspinal and spinal analgesia
|
|
|
subpopulations of mu receptors
|
mu-1: activation is speculated to be responsible for analgesia
mu-2: responsible for hypoventilation, bradycardia, and physical dependence |
|
|
all responses evoked by mu-1
|
-supraspinal analgesia
-spinal analgesia -euphoria -miosis -urinary retention |
|
|
specific responses due to mu-2 receptors
|
-spinal analgesia
-depression of ventilation -bradycardia -constipation -physical dependence |
|
|
specific responses to kappa receptors
|
-supraspinal analgesia
-spinal analgesia -dysphoria -sedation -miosis |
|
|
specific responses to delta receptor activation
|
-supraspinal analgesia
-spinal analgesia -depression of ventilation -urinary retention -physical dependence |
|
|
what happens in terms of opioids with advancing age?
|
after about 60yo, there is decreasing sensitivity to pain, and enhanced analgesic response to opioids
|
|
|
neuraxial opioids
|
opioids can be placed in the epidural or subarachnoid space in order to activate opioid receptors (principally mu receptors) in the substantia gelatinosa of the spinal cord
|
|
|
how does analgesia produced by neuraxial opioids differ from that of IV opioids or reginal infiltration of local anesthetic?
|
neuraxial opioids are not associated with sympathetic nervous system denervation, skeletal muscle weakness, or loss of proprioception
|
|
|
analgesia produced by neuraxial opioids
|
is dose-related, and is specific for visceral rather than somatic pain
|
|
|
epidural vs. subarachnoid dose
|
the epidural dose is 5-10x the subarachnoid dose
|
|
|
what does analgesia following epidural placement of opioids reflect?
|
diffusion of the drug across the dura to gain access to mu opioid receptors on the spinal cord, and systemic absorption to produce effects similar to IV opioids
|
|
|
speed of onset of analgesia for epidural opioids
|
is related to lipid solubility, with poorly lipid-soluble opioids such as morphine being much slower but with a longer duration of action than lipid-soluble opioids like fentanyl
|
|
|
what happens to opioids placed in the epidural space?
|
undergo significant systemic absorption and passage into the subarachnoid space
|
|
|
again, what influences penetration of the dura by opioids?
|
lipid solubility, fentanyl and sufentanil pass much more rapidly than morphine
|
|
|
blood concentrations of opioids after epidural injection
|
epidural injection of fentanyl, sufentanil, and morphine produce blood concentrations comparable to IM injection of equivalent doses
|
|
|
vascular absorption of intrathecal opioids
|
is insignificant
|
|
|
what affects cephalad spread of opioids in the CSF?
|
lipid solubility:
-fentanyl and sufentanil are lipid-soluble and thus will have limited cephalad migration due to uptake into the spinal cord -morphine is less lipid-soluble and will remain in the CSF for transfer to more cephalad locations |
|
|
how do patient actions and positioning affect opioid spread in the CSF?
|
-coughing and straining will affect movement
-patient positioning will not affect movement |
|
|
what are the side effects of neuraxial opioids due to?
|
the presence of the drugs in the CSF and/or systemic circulation
|
|
|
most common side effects of neuraxial opioids
|
-pruritus
-nausea and vomiting -urinary retention -depression of ventilation -sedation |
|
|
depression of ventilation due to neuraxial opioids
|
can get early or late depression of ventilation
|
|
|
early depression of ventilation due to neuraxial opioids
|
occurs within 2 hours of neuraxial injection
|
|
|
late depression of ventilation due to neuraxial opioids
|
typically occurs 6 to 12 hours after epidural or intrathecal injection of morphine, and is due to cephalad spread of the morphine and interaction with opioid receptors in the ventral medulla
|
|
|
factors that increase the risk of delayed depression of ventilation
|
-high dose opioids
-low lipid solubility opioids -concomitant use of either IV or parenteral opioids, or other sedatives -lack of opioid tolerance -advanced age |
|
|
it can be difficult to detect depression of ventilation induced by neuraxial opioids, what is perhaps the most reliable sign, and what are some others?
|
-most reliable is perhaps depressed level of consciousness, likely due to hypercarbia
others include: -low pulse ox indicating arterial hypoxemia, though supplemental oxygen easily treats this -low respiratory rate (<8) |
|
|
what is the effective treatment of depression of ventilation produced by neuraxial opioids?
|
naloxone
|
|
|
what is the prototype opioid agonist?
|
morphine
|
|
|
effects of morphine in humans
|
analgesia, euphoria, sedation, and a decreased ability to concentrate
|
|
|
morphine effect on pain
|
the cause of the pain persists, but even small doses of morphine increase the threshold to pain, and modify the perception of noxious stimulation so it is no longer experienced as pain
|
|
|
which type of pain is best taken care of by morphine?
|
dull continuous pain is relieved better than intermittent sharp pain
|
|
|
morphine in contrast to nonopioid analgesics
|
it is good against pain arising from viscera, as well as from skeletal muscles, joints, and pain from intuguement
|
|
|
when is analgesia with morphine most prominent?
|
when it is given before the painful stimulus occurs (preemptive analgesia)
-this is pertinent because should consider giving opioids before acute surgical stimulus begins |
|
|
what may happen if morphine is given in the absence of pain?
|
may get dysphoria rather than euphoria
|
|
|
clearance of opioids (in general)
|
is principally by hepatic metabolism, but differences in opioid's lipid solubility accounts for the pharmacokinetic differences seen between the opioids
|
|
|
morphine routes of administration
|
can be given IM, orally, or IV. Absorption from the GI tract is unpredictable, so orally administered morphine is as well. IM porphine is well-absorbed, and obviously most morphine in the perioperative period is given IV so it bypasses the unpredictability of enteral.
|
|
|
IM morphine onset and peak effects
|
-morphine is well-absorbed from IM injections
-get onset of effect in 15 to 30 min -peak effect in 45-90 min |
|
|
what is the duration of action of IM morphine?
|
4 hours
|
|
|
peak effect of IV morphine
|
-the peak effect, meaning equilibration time between the blood and brain, is delayed for morphine compared to other opioids such as fentanyl or alfentanil
-takes 15-30 min |
|
|
IV morphine in the CNS
|
-only a small amount gains access to the CNS, with estimates being that <0.1% of IV morphine reaches the CNS at the time of its peak effect
|
|
|
reasons for poor morphine penetration into the CNS
|
-poor lipid solubility
-rapid conjugation (metabolism) with glucuronic acid |
|
|
morphine metabolites
|
there are 2:
-morphine-3-glucuronide -morphine-6-glucuronide |
|
|
which is active and which is inactive?
|
morphine-3-glucuronide is inactive, while morphine-6-glucuronide is active and may produce analgesia and depression of ventilation
|
|
|
1 other consideration with morphine's metabolites
|
morphine glucuronides are eliminated renally, so elimination may be impaired in renal failure patients causing the metabolites to accumulate, resulting in unexpected depression of ventilation
|
|
|
morphine effect on CV system in supine patients
|
-morphine, even when given in large doses such as 1 mg/kg IV to supine normovolemic pts is not likely to produce direct myocardial depression or hypotension
|
|
|
conversely, morphine effects on the CV system in other patients
|
-in patients changing from sitting to standing it may produce orthostatic hypotension and syncope
|
|
|
what is this presumably due to?
|
drug-induced impairment of compensatory sympathetic nervous system responses
|
|
|
how else can morphine cause a decrease in BP?
|
due to drug-induced histamine release, especially after rapid IV administration of large doses (note: the synthetic and shirt-acting opioids do not cause histamine release)
|
|
|
do opioids sensitize the myocardium to catecholamines?
|
no
|
|
|
what happens when opioid agonists are given in combination with nitrous oxide or benzos?
|
get a decrease in systemic blood pressure which is not seen with the administration of any of those agents alone
|
|
|
opioids effect on ventilation
|
all cause a dose-dependent depression of ventilation, primarily through agonism of mu-2 receptors, leading to a direct depressant effect on the ventilation centers of the brainstem
|
|
|
how is the depression of ventilation by opioids characterized?
|
-decreased responsiveness of the ventilatory centers to CO2, which is reflected by an inc in PaCO2 and displacement of the CO2 curve to the right
|
|
|
length of ventilatory depression follwing opioids
|
lasts for several hours
|
|
|
effect of high doses of opioids on ventilation
|
may produce apnea, although the patient will still be awake and able to take a breath if asked to do so
|
|
|
how is the effect of opioids on ventilation manifested clinically?
|
by a decreased frequency of breaths, with a compensatory inc in tidal volume
|
|
|
effect of all opioids on consciousness
|
even in high doses, do not reliably produce unconsciousness, especially in young patients, which emphasizes that they are not true anesthetics
|
|
|
effect of opioids on cerebral circulation
|
-in the absence of hypoventilation they act as cerebral vasoconstrictors, so decrease CBF and possilby ICP
-however, have to be careful in pts with head injury as the depression of ventilation caused by opioids and subsequent accumulation of CO2 will cause an unwanted inc in ICP |
|
|
opiods effect on EEG and neuromuscular blocking drugs
|
-do not produce seizure activity on EEG
-do not interfere with neuromuscular blocking drugs |
|
|
what can be seen with larger doses of opioids, especially if they are given rapidly?
|
-skeletal muscle rigidity, especially of the thoracoabdominal muscles, which can be severe enough to interfere with ventilation and oxygenation
|
|
|
how should this "stiff-chest syndrome" be dealt with?
|
by giving a NMB or opioid antagonist
|
|
|
what is the miosis caused by opioids caused by?
|
their excitatory action on the oculomotor nerve
|
|
|
quantification of the contribution of opioid to total anesthetic requirments
|
-can be quantified by looking at the decrease in MAC of volatiles when opioids are present
-they ahve been shown to dec MAC in a dose-dependent manner up to a ceiling (about a 50% dec in MAC), after which no further dec will be seen with increasing doses of opioids |
|
|
what does this last observation imply?
|
that it is doubtful opioid agonists can provide total amnesia reliably in every patient, even in high doses
|
|
|
opioid effect on biliary smooth muscle
|
-will cause spasm of biliary smooth muscle, resulting in an increase in intrabiliary pressure causing epigastric distress and biliary colic, which may be mistaken for angina
|
|
|
how can this pain be differentiated from angina?
|
-giving nitroglycerine will relieve the pain from both myocardial ischemia and biliary spasm, but naloxone will only relieve the pain from biliary spasm, not myocardial ischemia
|
|
|
opioid effect on the GI tract
|
-opioids, especialy morphine, cause decreased peristaltic activity, and increased tone of the pyloric sphincter causing delayed gastric and intestinal emptying
|
|
|
opioid effect on the bladder
|
will increase bladder sphincter tone, making it difficult to spontaneously urinate
|
|
|
what is the cause of opiod incuded nausea and vomtiing?
|
durect stimulation of dopamine receptors in the chemoreceptor trigger zone in the floor of the 4th ventricle
|
|
|
what is supporting evidence for activation of these dopamine receptors as the mechanism of opiod induced nausea and vomiting?
|
the antiemetic efficacy of dopamine antagonists, such as the butyrophenones
|
|
|
what else does morphine do centrally in terms of emesis?
|
it depressed the vomiting center of the medulla
|
|
|
what is the consequence of this and of the activation of receptors in the chemoreceptor trigger zone?
|
there is less nausea and vomiting with morphine given IV than IM, since IV morphine reaches both the chemoreceptor trigger zone and the vomiting center in the medulla (which it depresses) with equal speed, whereas not with IM
|
|
|
what happens when morphine is given to recumbent patients, and what does this suggest?
|
it is relatively uncommon, suggesting a vestibular component might contribute to opioid-induced nausea and vomiting
|
|
|
what are the 2 major limitations to the clinical use of opioids?
|
tolerance and dependence
|
|
|
tolerance
|
the development of the need to increase the dose of opioid to achieve the same analgesic effect previously achieved by a lower dose
|
|
|
how long does it usually take tolerance to develop?
|
2-3 weeks, although continuous IV infusions of opioids in pain-free animals have shown a profound decrease in analgesia in 2-8 hours
|
|
|
which specific effects of opioids persist, and which do not with tolerance?
|
-the miotic and constipating effects persist
-the depression of ventilation does not |
|
|
opioid dependence
|
-addiction
-when the opioid is withdrawn, a typical withdrawl abstinence syndrome of diaphoresis, insomnia, restlessness, and abdominal cramps occurs within 15-20 hours, with a peak in 2-3 days |
|
|
opioids and the immune system
|
-opioids and endogenous opioid peptides modulate the immune system, as shown by alterates in things like neutrophil chemotaxis, phagocytic activity, and the secretion of cytokines
-a link is provided between pain and immunity by the fact that the PNS and CNS are involved in immune function |
|
|
meperidine
|
a synthetic opioid that is 1/10 as potent as morphine
|
|
|
what is meperidine structurally similar to?
|
atropine, it even has a mild stropine-like antispasmodic effect
|
|
|
what happens well with meperidine that doesnt with morphine?
|
meperidine is well absorbed from the GI tract
|
|
|
meperidine metabolites
|
-the principle metabolite is normeperidine, and it produces CNS stimulation at high concentrations
|
|
|
what is unique about meperidine among opioids, and how is this thought to happen?
|
it is uniquely effective at suppressing post-op shivering, which is thought to be due to stimulation of kappa receptors
|
|
|
differences in CV system effects of meperidine vs. morphine
|
-with meperidine, orthostatin hypotension is more frequent and profound
-meperidine, unlike morphine, rarely causes bradycardia, but may cause an increase in HR similar reflecting its atropine-like effects |
|
|
what do large doses of meperidine result in (also unique among opioids)?
|
decreases in myocardial contractility
|
|
|
fentanyl
|
a synthetic ipioid agonist structurally related to meperidine
|
|
|
potency of fentanyl
|
75-125 more powerful than morphine
|
|
|
onset and duration of action of fentanyl vs. morphine
|
IV fentanyl has a more rapid onset and shorter duration of action than morphine
|
|
|
metabolism of fentanyl
|
is a substrate for the CYT P450 enzymes (CYP3A), and is susceptible to drug interactions that reflect interference with enzyme activity (though this is less likely than with alfentanil)
|
|
|
onset of fentanyl
|
despite the impression that it has such a rapid onset, there is a distinct time lag between the peak plasma concentration and the peak slowing on the EEG, which reflects the effect-site equilibrium time between the blood and brain, which is 6.4 min
|
|
|
why is fentanyl more potent and shorter acting than morphine?
|
-the greater potency and more rapid onset reflect greater lipd solubility which facilitates passage across the blood brain barrier
-the short duration of action reflects rapid redistribution to inactive tissues like fat and skeletal muscles, with associated dec in plasma concentration |
|
|
multiple IV doses/infusion of fentanyl
|
during either of these there is progressive saturation of inactive tissue sites, causing the plasma concentration of the drug to NOT drop rapidly, so the duration of analgesia and the depression of ventilation may be prolonger
|
|
|
context sensitive half-time of fentanyl
|
when the duration of continuous infusion increases beyond 2 hours, the CSHT becomes greater than sufentanyl
|
|
|
side effects of fentanyl vs. morphine
|
resemble the side effects of morphine, except that histamine release and hypotension dont occur, even with rapid IV boluses
|
|
|
other side effects of fentanyl
|
-prior administration of a benzo, and possibly other IV and inhaled anesthetics, alters the CV response to fentanyl, so that bradycardia becomes prominent when fentanyl is given
|
|
|
fentanyl with benzos
|
-analgesic doses of fentanyl greatly potentiate the effects of other depressant/sedatives (ie. benzos), causing marked synergism of hypnosis and depression of ventilation
|
|
|
other drug interactions with fentanyl
|
any drugs that inhibit the CYP4503A4 enzymes, such as the protease inhibitor ritonavir, can delay clearance of fentanyl, resulting in prolonged effects of the opioid
|
|
|
uses of fentanyl
|
can be administered in a wide range of doses for a wide range of effects
|
|
|
low doses of fentanyl
|
(1-2 ug/kg IV)
-are used to provide analgesia -if given before surgical stimulation may decrease the subsequent amount necessary (preemptive analgesia) |
|
|
medium dose fentanyl
|
(2-20 ug/kg IV)
-may be given as an adjuvant to inhaled anesthetics to blunt circulatory responses to direct laryngoscopy for tracheal intubation, or sudden changes to surgical stimulation |
|
|
opioids given after surgical stimulation
|
-are frequently not useful intraoperatively after surgical stimulation has already evoked systemic hypertension
-though intraop tachycardia may respond to small IV doses |
|
|
what should you consider in terms of the timing for treating things such as intraoperative tachycardia?
|
the effect-site equilibrium time, which for fentanyl is 6.4 minutes (and is prolonged when compared to alfentanil and remifentanil)
|
|
|
high dose fentanyl
|
(50-150 ug/kg IV)
-can be used alone to produce surgical anesthesia |
|
|
what is the advantage of using opioids for surgical anesthesia?
|
they give stable hemodynamics
|
|
|
what is the disadvantage?
|
possible patient awareness, as opioids are not complete anesthetics
|
|
|
other routes of administration of fentanyl
|
can be given intranasally or transdermally
|
|
|
sufentanil
|
a fentanyl analog, which is 5-10x as potent as fentanyl, and 500-1000x as potent as morphine
|
|
|
effect-site equilibrium time of sefentanil
|
similar to fentanyl, about 6.2 min
|
|
|
termination of effect of sufentanil
|
-small doses are temrinated by rapid redistribution to inactive tissues
-large or repeated doses can result in a cumulative effect; after an infusion is terminated the plasma concentration decrease is accelerated by metabolism and continued redistribution to peripheral compartments |
|
|
sufentanil context sensitive half-time
|
-is less than alfentanil for infusions up to 8 hours
|
|
|
context sensitive half-time of opioids
|
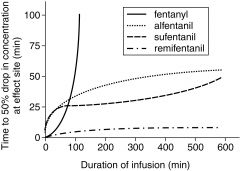
|
|
|
how do the effects of sufentanil compare to fentanyl?
|
depression of ventilation and bradycardia may be more profound with sufentanil
|
|
|
again, what can large doses of potent opioids, such as sufentanil, cause?
|
skeletal muscle rigidity, especially of the thoracic muscles, possibly making ventilation difficult
|
|
|
alfentanil
|
-fentanyl analog
-1/5-1/10 as potent, with 1/3 the diration of action of fentanyl |
|
|
what is the unique advantage of alfentanil over fentanyl or sufentanil?
|
the more rapid onset of action (effect-site equilibrium is about 1.4 min)
|
|
|
why is alfentanil quicker?
|
-due to its low pK, so almost 90% of it exists in the lipid-soluble nonionized form at physiologic pH
|
|
|
consequences of the rapid peak effect of alfentanil
|
makes it useful to blunt the response of a single, brief stimulus, such as tracheal intubation, or retrobulbar block
|
|
|
clearance of alfentanil
|
-there is a 10-fold variation in interindividual systemic clearance of alfentanil as reflected in showing widely varying plasma concentrations after fixed doses
-likely reflects differences in CYP3A4 enzyme activity between individuals |
|
|
remifentanil
|
-opioid with potency similar to fentanyl (250x morphine, vs. 75-125 for fentanyl)
|
|
|
remifentanil effect-site equilibrium time
|
similar to alfentanil, is about 1.2 min
|
|
|
structure of remifentanil
|
-is unique among opioids because of its ester linkage, which makes it susceptible to hydrolysis by nonspecific plasma and tissue esterases to inactive metabolities
(note these are different from pseudocholinesterase which hydrolyzes sux and mivacurium) |
|
|
consequences of this structure
|
gives remifentanil brevity of action, precise and rapid titratability, noncumulative effects, and a rapid recovery after IV infusion is discontinued
|
|
|
context sensitive half-time of remifentanil
|
is independent of the duration of the infusion, and is about 4 minutes
|
|
|
uses of remifentanil
|
-can be used for induction, giving 1 ug/kg IV over 60 to 90 seconds
-can be used as the analgesic component of general anesthesia, giving 0.5-2 ug/kg/min IV, or as a sedation technique with this |
|
|
what should you do when stopping remi?
|
give a long-acting opioid to ensure analgesia once remi has quickly worn off
|
|
|
remifentanil combined with what is especially synergistic?
|
when combined with propofol, resulting in severe depression of ventilation
|
|
|
how can remifentanil be effectively used for labor and delivery?
|
intermittent administration as PCA
|
|
|
what unwanted effect can occur after remifentanil infusion?
|
-analgesic requirements postop in patients getting remifentanil intraop are surprisingly high, suggesting it may cause acute opioid tolerance (though not all data supports this)
|
|
|
opioid agonist-antagonists
|
-drugs that bind to mu receptors where they produce limited responses (partial agonists) or no effect (antagonist)
-they often exert partial agonist effects at kappa and delta receptors |
|
|
specific opioid agonist-antagonists
|
include:
-pentazocine -butorphanol -nalbuphine -buprenorphine -nalorphine |
|
|
what are the positives and negatives of the antagonist properties of these?
|
-they lessen the efficacy of subsequently administered drugs
-but, they also have the advantage of providing postop analgesia with the hope of less associated depression of ventilation |
|
|
side effects of opioid agonist-antagonists
|
are similar to opioids, and in addition they may cause dysphoric reactions
|
|
|
advantages of opioid agonist-antagonists
|
can produce analgesia with minimal depression of ventilation, and with less physical dependence
|
|
|
dosing of opioid agonist-antagonists
|
exhibit a ceiling effect, with increasing doses beyond a certain point not producing any other pharmacologic effects
|
|
|
opioid antagonists
|
-minor changes to the structure of opioid agonists can make drugs opioid antagonists
-they have high affinity for opioid receptors, resulting in displacement of opioid agonists from mu receptors, without activation of the mu receptors |
|
|
specific list of opioid antagonists
|
-naloxone
-nalmefene -naltrexone |
|
|
naloxone dose
|
1-4 ug/kg IV
|
|
|
duration of action of naloxone
|
-is short, 30-45 min, and may result in its rapid removal from the brain with return of opioid effects unless more doses of naloxone are given
|
|
|
alternative administration form of naloxone
|
can be given as a continuous infusion (3-5 ug/kg/hr IV)
|
|
|
side effects of naloxone
|
are basically the result of increased sympathetic activity due to the reversal of analgesia and sudden perception of pain; include:
-tachycardia -systemic hypertension -pulmonary edema -cardiac dysrhythmias, including V-fib |
|
|
bottom line in terms of pK of opioids
|
alfentanil is the lowest, and remifentanil is also relatively low, resulting in their rapid effect-site equilibrium
|
|
|
bottom line in terms of percent of nonionization of opioids
|
alfentanil has the highest percentage, resulting in the same thing as above
|
|
|
context sensitive half-times of the fentanyl derivatives
|
fentayl: 6.8 min
sufentanil: 6.2 alfentanil: 1.4 remifentanil: 1.1 |
|
|
onset, peak intensity, and duration of morphine withdrawal
|
onset: 6-18 hours
peak intensity: 36-72 hours duration: 7-10 days |
|
|
onset, peak intensity, and duration of heroin withdrawal
|
onset: 6-18 hours
peak intensity: 36-72 hours duration: 7-10 days |
|
|
onset, peak intensity, and duration of meperidine withdrawal
|
onset: 2-6 hours
peak intensity: 8-12 hours duration: 4-5 days |
|
|
onset, peak intensity, and duration of fentanyl withdrawal
|
onset: 2-6 hours
peak intensity: 8-12 hours duration: 4-5 days |
|
|
onset, peak intensity, and duration of methadone withdrawal
|
onset: 24-48 hours
peak intensity: 3-21 days duration: 6-7 weeks |

