![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
285 Cards in this Set
- Front
- Back
|
How do neuromuscular blocking drugs work?
|
They interrupt transmission of nerve impulses at the neuromuscular junction and thereby produce paresis or paralysis of skeletal muscles
|
|
|
Classification of neuromuscular blockers
|
Are classified on the basis of electrophysiologic differences in their mechanisms of action and duration of action as depolarizing and nondepolarizing, the latter of which are further subdivided into long, intermediate, and short-acting drugs
|
|
|
General mechanism of action of depolarizing versus nondepolarizing neuromuscular blocking drugs
|
Depolarizing drugs mimic the actions of acetylcholine, while nondepolarizing drugs interfere with the actions of acetylcholine
|
|
|
What is the only depolarizing neuromuscular blocker used clinically?
|
Succinylcholine
|
|
|
What else is unique about succinylcholine?
|
It is also the only neuromuscular blocking drug with both a rapid onset and ultrashort duration of action
|
|
|
Which nondepolarizing neuromuscular blocker most closely resembles the onset characteristics of succinylcholine?
|
Rocuronium
|
|
|
What are the principal clinical uses of neuromuscular blockers?
|
To produce skeletal muscle relaxation for facilitation of tracheal intubation and to provide optimal surgical working conditions
|
|
|
When might neuromuscular blocking drugs also be used?
|
In emergency rooms, cardiopulmonary resuscitation situations, and ICU settings to facilitate mechanical ventilation
|
|
|
What is it important to recognize about neuromuscular blocking drugs?
|
They lack analgesic or anesthetic effects and must not be used to render an inadequately anesthetized patient paralyzed
|
|
|
What must be done whenever significant skeletal muscle weakness is produced by these drugs?
|
Ventilation of the lungs must be mechanically provided
|
|
|
How is intraoperative evaluation of neuromuscular blockade clinically monitored?
|
By visually monitoring the mechanical response, which response, produced by electrical stimulation of the peripheral nerve, usually a branch of the ulnar or facial nerve, delivered from a peripheral nerve stimulator
|
|
|
Which are the long acting nondepolarizing neuromuscular blockers?
|
Pancuronium
|
|
|
Which are the intermediate – acting neuromuscular blockers?
|
Vecuronium, rocuronium, atracurium, cisatracurium
|
|
|
Which are the short – acting neuromuscular blockers?
|
Mivacurium
|
|
|
What influences the choice of neuromuscular blocker?
|
It's speed of onset, duration of action, route of elimination, and associated side effects such as drug – induced changes and systemic arterial blood pressure or heart rate, or both
|
|
|
When is succinylcholine a good choice?
|
When tracheal intubation is the reason for administering the drug, because of its rapid onset and brief duration of skeletal muscle paralysis
|
|
|
Use of rocuronium
|
Can also be used to facilitate tracheal intubation because of its rapid onset time, but it's duration of action is much longer
|
|
|
When are nondepolarizing neuromuscular blockers usually selected?
|
One longer periods of neuromuscular blockade are needed they are usually selected, even though succinylcholine can technically be administered as a continuous IV infusion
|
|
|
When is administration of long – or intermediate – acting nondepolarizing neuromuscular blockers used?
|
When rapid onset of skeletal muscle paralysis is not necessary
|
|
|
What does the neuromuscular junction consist of?
|
A pre-junctional motor nerve ending separated from the highly folded postjunctional membrane of the skeletal muscle by synaptic cleft
|
|
|
What three cells constitute the synapse?
|
The motor neuron (i.e. nerve terminal), muscle fiber, and Schwan's cell
|
|
|
What innervates the muscle?
|
The motor neuron from the ventral one of the spinal cord
|
|
|
How many synapses does each muscle fiber receive?
|
Only one
|
|
|
Motor nerve terminal
|
The motor nerve loses it's myelin and terminates on the muscle fiber, the nerve terminal is covered by a Schwann cell, and has vesicles clustered about the membrane thickening which are the active zones, toward its synaptic side and mitochondria and microtubules toward its other side
|
|
|
Synaptic cleft
|
A synaptic gutter made up of primary and many secondary clefts, separates the nerve from the muscle
|
|
|
The muscle surface at the neuromuscular junction
|
Is corrugated, and dense areas on the shoulders of each fold contain acetylcholine receptors; sodium channels are present throughout the clefts and throughout the muscle membrane
|
|
|
Process of neuromuscular transmission
|
Neuromuscular transmission is initiated by the arrival of an impulse at the motor nerve terminal with an associated influx of calcium ions and resultant release of the ligand acetylcholine, acetylcholine then binds to nicotinic cholinergic receptors (the ligand – gated channel) on post junctional membranes and thereby causes a change in membrane permeability to ions, principally potassium and sodium, this change in permeability and movement of ions causes a decrease in the transmembrane potential from about -90 mV to -45 mV (threshold potential), at which point a propagated action potential spreads over the surfaces of skeletal muscle fibers and leads to muscular contraction
|
|
|
Termination of neuromuscular junction action
|
Acetylcholine is rapidly hydrolyzed, within 15 ms, by the enzyme acetylcholinesterase (true cholinesterase), thus restoring membrane permeability (repolarization) and preventing sustained depolarization
|
|
|
Where is acetylcholinesterase primarily located?
|
In the folds of the end – plate region, which places it in close proximity to the site of action of acetylcholine
|
|
|
Acetylcholine storage and release
|
Acetylcholine is synthesized in the motor nerve terminal and the protein synapsin anchors the acetylcholine vesicle to the release site of the terminal, some of the acetylcholine is been released and the rest is held in reserve for response to a stimulus. Presynaptic receptors, aided by calcium, facilitate replenishment of the motor nerve terminal
|
|
|
What can stimulate or depress the presynaptic receptors that facilitate replenishment of the motor nerve terminal?
|
Succinylcholine and neostigmine can stimulate them, small doses of nondepolarizing neuromuscular blocking drugs can depress them
|
|
|
Structure of post-junctional receptors
|
Postjunctional receptors are glycoproteins which consist of five subunits, 2A1 Beta one Gamma and 1D, which are arranged such that the channels form that allows the flow of ions along the concentration gradient across the cell membrane, which is the basis of normal neuromuscular transmission
|
|
|
Structure of extra junctional receptors
|
Will retain the two alpha subunits but may have an altered gamma or delta subunit by the substitution of an epsilon unit
|
|
|
Where do neuromuscular blocking drugs bind?
|
The two alpha subunits of the post-junctional receptor are the binding sites for acetylcholine and neuromuscular blocking drugs
|
|
|
Nondepolarizing neuromuscular blocker binding
|
Occupational one or both of the subunits by a nondepolarizing neuromuscular blocker will cause the ion channel to remain closed, and ion flow to produce depolarization cannot occur
|
|
|
Binding of succinylcholine
|
Succinylcholine attaches to alpha sites and causes behind general to remain open (mimics acetylcholine) thereby resulting in prolonged depolarization
|
|
|
What happens at large doses of nondepolarizing neuromuscular blockers?
|
At these doses they may also act to include the channel and in this way prevent the normal flow of ions
|
|
|
What is different about neuromuscular blockade secondary to occlusion of the channels?
|
It is resistant to drug – enhanced antagonism with anti-cholinesterase drugs
|
|
|
How can the lipid environment around cholinergic receptors be altered and what does this do to ion channels?
|
It can be altered by drugs such as volatile anesthetics, and it changes the properties of the ion channels
|
|
|
Extra junctional receptors
|
Whereas post-junctional receptors are confined to the area of the end plate precisely opposite the pre-junctional receptors, extra junctional receptors (with the epsilon unit replacing the gamma units) are present throughout skeletal muscles
|
|
|
Synthesis at extra junctional receptors
|
Is normally suppressed by neural activity, however prolonged inactivity, sepsis, and denervation or trauma to skeletal muscles such as burn injury may be associated with proliferation of extra junctional receptors
|
|
|
Activation of extra junctional receptors
|
When activated they stay open longer and permit more ions to flow, which in part explains the exaggerated hyperkalemic response when succinylcholine is given to patients with denervation or burn injury
|
|
|
What else does proliferation of these receptors account for?
|
The resistance or tolerance to nondepolarizing neuromuscular blocking drugs which occurs with burns or prolonged (several days) mechanical ventilation of the lungs
|
|
|
Structure of neuromuscular blocking drugs
|
Neuromuscular blocking drugs are quaternary ammonium compounds that have at least one positively charged nitrogen atom that binds to the alpha – subunit of postsynaptic cholinergic receptors
|
|
|
What are these drugs structurally similar to?
|
The endogenous neurotransmitter acetylcholine, for example succinylcholine is two molecules of acetylcholine linked by methyl groups
|
|
|
Advantage of the structure of acetylcholine
|
The long, slender, flexible structure of acetylcholine allows it to bind to and activate cholinergic receptors
|
|
|
Advantage of the structure of nondepolarizing neuromuscular drugs
|
They are bulky rigid molecules that, although containing portions similar to acetylcholine, do not activate cholinergic receptors
|
|
|
What are the two types of nondepolarizing neuromuscular blocking drugs?
|
They are either aminosteroid compounds or benzylisoquinolinium compounds
|
|
|
Which nondepolarizing neuromuscular blockers are aminosteroid?
|
Pancuronium, vecuronium, rocuronium
|
|
|
Which neuromuscular blocking drugs are benzylisoquinolinium compounds?
|
Atracurium, cisatracurium, mivacurium
|
|
|
Which drug is the bisquaternary aminosteroid neuromuscular blocking drugs most closely related to acetylcholine structurally?
|
Pancuronium
|
|
|
What does this mean practically for pancuronium?
|
The acetylcholine – like fragments of pancuronium give the steroid molecule it's high degree of neuromuscular blocking activity
|
|
|
How are vecuronium and rocuronium related to pancuronium?
|
They are monoquaternary analogues of pancuronium
|
|
|
Do aminosteroid neuromuscular blocking drugs have hormonal activity?
|
No
|
|
|
What are benzylisoquinolinium derivatives more likely to do then aminosteroid derivatives, and what does this reflect?
|
They are more likely to evoke the release of histamine, presumably reflecting the presence of a tertiary amine
|
|
|
What is unique about succinylcholine?
|
It is the only depolarizing neuromuscular blocker use clinically, and the only one with both a rapid onset and ultrashort duration of action
|
|
|
Typical dose of succinylcholine
|
0.5 to 1.5 mg/kilogram IV
|
|
|
Onset and duration of action of succinylcholine
|
Produces rapid onset of skeletal muscle paralysis in 30 to 60 seconds, which lasts 5 to 10 min.
|
|
|
What do these characteristics make succinylcholine ideal for?
|
For providing rapid skeletal muscle paralysis to facilitate tracheal intubation; succinylcholine has been used clinically for over 50 years and despite consistent efforts no drug has been developed which is better for tracheal intubation
|
|
|
What happens if a sub paralyzing dose of a nondepolarizing neuromuscular blocker is administered 2 to 4 min. before injection of succinylcholine to blunt fasciculations?
|
(Pretreatment with 5% to 10% of its 95% effective dose [ED95])
the dose of succinylcholine should be increased by about 70% |
|
|
What is the downside the succinylcholine?
|
It has many adverse effects
|
|
|
Mechanism of action of succinylcholine
|
Succinylcholine mimics the action of acetylcholine and produces a sustained depolarization of the post-junctional memory, and skeletal muscle paralysis occurs because it depolarized post-junctional membrane and in activated sodium channels cannot respond to subsequent release of acetylcholine (hence the designation depolarizing neuromuscular blocker)
|
|
|
What is depolarizing neuromuscular blockade also referred to as?
|
Phase 1 blockade
|
|
|
Phase 2 blockade
|
Present when the post-junctional membrane has become re-polarized but still does not respond normally to acetylcholine
|
|
|
Mechanism of phase 2 blockade
|
Is unknown but may reflect the development of non-excitable areas around the end plates that become repolarized but nevertheless prevent the spread of impulses initiated by the action of acetylcholine
|
|
|
With the initial dose of succinylcholine what begins to appear?
|
Subtle signs of phase 2 blockade, fade to tetanic stimulation
|
|
|
What predominates when the dose of succinylcholine exceeds 3 to 5 mg/kilogram IV?
|
Phase 2 blockade
|
|
|
What does phase 2 blockade resemble?
|
The blockade produced by nondepolarizing neuromuscular blockers
|
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
effect of administration of rocuronium |
Succinylcholine
Phase 1: antagonize phase 2: augment rocuronium: augment |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
administration of succinylcholine |
Succinylcholine
phase 1: augment phase 2: augment rocuronium: antagonize |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
administration of neostigmine |
Succinylcholine
phase 1: augment phase 2: antagonize rocuronium: antagonize |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
fasciculations |
Succinylcholine
phase 1: yes rocuronium: no |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
response to single electrical stimulation (single twitch) |
Succinylcholine
phase 1: decrease phase 2: decreased rocuronium: decreased |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
train – of – four ratio |
Succinylcholine
phase 1: more than 0.7 phase 2: less than 0.3 rocuronium: less than 0.3 |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
response to continuous (tetanus) electrical stimulation |
Succinylcholine
phase 1: sustained phase 2: unsustained rocuronium: unsustained |
|
|
Succinylcholine (phase 1 and 2) vs. rocuronium
Post – tetanic fasciculations |
Succinylcholine
phase 1: no phase 2: yes rocuronium: yes |
|
|
How was the sustained depolarization produced by the initial administration of succinylcholine initially manifested?
|
As transient generalized skeletal muscle contractions known as fasciculations
|
|
|
What is the sustained opening of sodium channels produced by succinylcholine associated with?
|
Leakage of potassium from the interior of cells sufficient to increased plasma concentrations of potassium by 0.5 mEq/liter
|
|
|
What can occur with proliferation of extra junctional receptors and damaged muscle membranes?
|
Many more channels will leak potassium and thereby lead to acute hyperkalemia
|
|
|
Metabolism of succinylcholine
|
Is hydrolyzed to inactive metabolites by plasma cholinesterase (pseudocholinesterase) produced in the liver
succinylcholine--> (plasma cholinesterase) succinylmonocholine+choline--> (plasma cholinesterase) succinic acid + choline |
|
|
Activity of succinylmonocholine
|
Has 1/20 to 1/80 the activity of succinylcholine at the neuromuscular junction
|
|
|
Activity of plasma cholinesterase
|
Has an enormous capacity to hydrolyze succinylcholine at a rapid rate, acetylcholine is metabolized even more rapidly, such that only a small fraction of the original IV dose reaches the neuromuscular junction
|
|
|
Activity of plasma cholinesterase at the neuromuscular junction
|
Plasma cholinesterase is not present at the neuromuscular junction
|
|
|
How then is succinylcholine's action terminated at the neuromuscular junction?
|
By its diffusion away from the neuromuscular junction into extracellular fluid
|
|
|
Therefore, how does plasma cholinesterase influence the duration of action of succinylcholine?
|
By controlling the amount of succinylcholine that is hydrolyzed before reaching the neuromuscular junction
|
|
|
Liver disease and succinylcholine
|
Liver disease must be severe before decreases in the synthesis of plasma cholinesterase are sufficient to prolong the effects of succinylcholine
|
|
|
Anti-cholinesterases
|
Phone anti-cholinesterases, as used in the treatment of myasthenia gravis and certain chemotherapeutic drugs (nitrogen mustard, cyclophosphamide) may so decreased plasma cholinesterase activity that prolonged skeletal muscle paralysis follows the administration of succinylcholine
|
|
|
Atypical plasma cholinesterase
|
A form of plasma cholinesterase which lacks the ability to hydrolyze ester bonds such as succinylcholine and mivacurium; its presence is often recognized only after an otherwise healthy patient experiences prolonged skeletal muscle paralysis, more than one hour, after the administration of a conventional dose of succinylcholine or mivacurium
|
|
|
How is subsequent diagnosis of the presence of a typical plasma cholinesterase determined?
|
By determination of the dibucaine number; dibucaine is an amide local anesthetic which inhibits normal plasma activity by about 80%, where is the activity of atypical enzyme is inhibited by only 20%
|
|
|
Dibucaine
|
And amide local anesthetic that inhibits normal plasma activity by about 80%, whereas the activity of atypical enzyme is inhibited by about 20%
|
|
|
Possible variants of plasma cholinesterase
|
Homozygous, typical
heterozygous homozygous, atypical |
|
|
Type of butylcholinesterase for each variant
|
Homozygous, typical: UU
heterozygous: UA homozygous, atypical: AA |
|
|
Incidence for each variant
|
Homozygous, typical: normal
heterozygous: 1/480 homozygous, atypical: 1/3200 |
|
|
Dibucaine number (percent inhibition of enzyme activity) for each variant
|
Homozygous, typical: 70 – 80
heterozygous: 50 – 60 homozygous, atypical: 20 – 30 |
|
|
Duration of succinylcholine – induced neuromuscular block in minutes for each variant
|
Homozygous, typical: five – 10
heterozygous: 20 homozygous, atypical: 60 – 180 |
|
|
Important thing to recognize about the dibucaine number
|
It reflects the quality of plasma cholinesterase, ability to metabolize succinylcholine and mivacurium, and not the quantity of the enzyme in the circulating plasma. For example decreases in plasma cholinesterase activity due to liver disease or anticholinesterases are often associated with the normal dibucaine number
|
|
|
When should succinylcholine usually not be given?
|
To patients 24 to 72 hours after major burns, trauma, and extensive denervation of skeletal muscles because it may result in acute hyperkalemia and cardiac arrest
|
|
|
What particular group should succinylcholine not be given to?
|
Apparently healthy boys who could possibly have unrecognized muscular dystrophy which would result in acute hyperkalemia and cardiac arrest, for this reason the FDA has issued a warning against the use of succinylcholine in children except for emergency control of the airway
|
|
|
Common adverse effects of succinylcholine
|
Cardiac dysrhythmias, fasciculations/myalgia, hyperkalemia, myoglobinuria, increased intraocular pressure, increased intra-gastric pressure, trismus
|
|
|
What cardiac dysrhythmias can follow administration of succinylcholine?
|
Sinus bradycardia, junctional rhythm, and even sinus arrest
|
|
|
What do these responses reflect about succinylcholine?
|
They reflect the action of succinylcholine at cardiac postganglionic muscarinic receptors where the drug mimics the normal effects of acetylcholine
|
|
|
When are cardiac dysrhythmias most likely to occur?
|
When the second IV dose of succinylcholine is administered about 5 min. after the first dose
|
|
|
What can be done to decrease the likelihood of the cardiac responses to succinylcholine?
|
IV administration of atropine or subparalyzing doses of nondepolarizing neuromuscular blocking drugs, pretreatment, 1 to 3 min. before succinylcholine administration
|
|
|
Intramuscular atropine in pretreatment of succinylcholine
|
Does not reliably protect against succinylcholine induced decreases in heart rate
|
|
|
What other effect will succinylcholine have form mimicking acetylcholine?
|
It will mimic the actions of acetylcholine at autonomic nervous system ganglia and may manifest as ganglionic stimulation with associated increases in systemic blood pressure and heart rate
|
|
|
What is the most serious complication possible succinylcholine?
|
Hyperkalemia sufficient to cause cardiac arrest, which may follow the administration of succinylcholine inpatients with unhealed skeletal muscle injury as produced by third – degree burns or trauma, or with denervation injury has caused by spinal cord transactions leading to skeletal muscle atrophy
|
|
|
Risk of hyperkalemia in these patients?
|
Increases with time and usually peaks 7 to 10 days after the injury; there is evidence that increased release of potassium after succinylcholine administration, with doses as small as 20 mg IV, can begin within 2 to 4 days after denervation injury
|
|
|
What other patients will extra junctional receptors and hyperkalemia develop?
|
In any patient who was immobile, for example critical care patients, for several days; cardiac arrest has occurred when succinylcholine has been used for emergency endotracheal intubation in the ICU
|
|
|
What is the duration of the susceptibility to the hyperkalemia affects of succinylcholine?
|
It is unknown, but the risk is probably decreased 3 to 6 months after denervation injury
|
|
|
Considering all factors one might it be prudent to avoid with administration of succinylcholine?
|
Administering succinylcholine to any patient more than 24 hours after a burn injury, extensive trauma, or spinal cord transaction
|
|
|
When presumably does the risk of hyperkalemia in burn victims diminish?
|
Presumably with healing of the skeletal muscles damaged by the burn injury, yet vulnerability to hyperkalemia is complicated and the common explanation of proliferation of extra junctional receptors thus providing more sites for potassium to leak outward across the cell membrane during depolarization is oversimplified and has not been proven to occur after burn injuries
|
|
|
Pretreatment with nondepolarizing neuromuscular blocking drugs
|
Minimally influences the magnitude of potassium release evoked by succinylcholine and cannot be relied upon as a safeguard
|
|
|
What other situations may place patients at risk for succinylcholine induced hyperkalemia?
|
Acute extensive trauma or upper motor neuron injuries
|
|
|
Does renal failure put patients at risk for exaggerated release of potassium?
|
No it does not, and succinylcholine can be safely administered to these patients assuming they do not have uremic neuropathy
|
|
|
Myalgias
|
Postoperative skeletal muscle myalgia manifest particularly in the muscles of the neck, back, and abdomen may follow the administration of succinylcholine, and it is speculated that unsynchronized contractions of skeletal muscle fibers during fasciculations associated with generalized depolarization lead to myalgias
|
|
|
How might myalgias localized to neck muscles be described by patients?
|
As a sore throat and may be incorrectly attributed to the previous presence of an endotracheal tube
|
|
|
In what patients are myalgias most likely occur?
|
In young adults undergoing minor surgical procedures which permit early ambulation
|
|
|
Prevention of myalgias
|
Pretreatment with nondepolarizing neuromuscular blockers can prevent fasciculations at subparalyzing doses, or prior administration of lidocaine can decrease the incidence but not totally prevent myalgia
|
|
|
Will magnesium help prevent myalgia?
|
Magnesium will help prevent fasciculations but not myalgias
|
|
|
What is an effective treatment for myalgia?
|
Nonsteroidal anti-inflammatory drugs
|
|
|
What does succinylcholine due to the eye?
|
Succinylcholine causes a maximum increase in intraocular pressure 2 to 4 min. after its administration, which is transient and lasts only 5 to 10 min.
|
|
|
Mechanism of intraocular pressure increase with succinylcholine
|
Is unknown, although contraction of extraocular muscles with associated compression of the globe has been presumed to contribute
|
|
|
What is the concern with increased intraocular pressure and succinylcholine?
|
That extraocular muscle contraction could cause extrusion of intraocular contents in the presence of an open eye injury, which has resulted in the common clinical practice to avoid succinylcholine in these patients
|
|
|
Validity of avoiding succinylcholine in patients with open eye injuries
|
The theory has never been substantiated and is challenged by the report of patients with an open eye injury in whom IV administration of succinylcholine did not cause extrusion of global contents, furthermore there is evidence that contraction of extraocular muscles does not contribute to the increasing intraocular pressure which accompanies succinylcholine administration
|
|
|
Effect of succinylcholine on intracranial pressure
|
Increases intracranial pressure do not occur consistently with succinylcholine
|
|
|
Effect of succinylcholine on intragastric pressure
|
Succinylcholine causes unpredictable increases in intragastric pressure, and when intragastric pressure does increase it seems to be related to the intensity of fasciculations, thus emphasizing the potential value of preventing this skeletal muscle activity by prior administration of a subparalyzing dose of a nondepolarizing neuromuscular drug
|
|
|
Problem with increasing intragastric pressure with succinylcholine
|
It is thought that increased intragastric pressure may cause passage of gastric fluid and contents into the esophagus and pharynx with the subsequent risk for pulmonary aspiration, although this hypothesis is unproven
|
|
|
What is the final adverse effect of succinylcholine?
|
Trismus
|
|
|
Trismus with succinylcholine administration
|
It is considered a normal response to get incomplete jar relaxation with messenger jaw rigidity after a halothane – succinylcholine sequence in children (occurs in about 4.4% of patients); in extreme cases this response may be so severe that the ability to mechanically open the patient's mouth is limited
|
|
|
What is the difficulty with trismus and succinylcholine administration?
|
The difficulty is in separating the normal response to succinylcholine from the master rigidity that can be associated with malignant hyperthermia, but because succinylcholine is not recommended for use in children trismus is less of an issue
|
|
|
How are nondepolarizing neuromuscular blocking drugs classified clinically?
|
As long, intermediate, or short acting
|
|
|
How do nondepolarizing neuromuscular blocking drugs work?
|
They act like competing with acetylcholine 4A – subunits at the postjunctional nicotinic cholinergic receptors and preventing changes in ion permeability, as a result depolarization cannot occur, hence the designation nondepolarizing blockade, and skeletal muscle paralysis develops
|
|
|
What does not occur with nondepolarizing muscle relaxants as opposed to depolarizing?
|
The skeletal muscle fasciculations seen with succinylcholine do not accompany the onset of nondepolarizing blockade
|
|
|
Pharmacokinetics of nondepolarizing neuromuscular blockers
|
Because of their quaternary ammonium they are highly ionized, water – soluble compounds at physiologic pH and possess limited lipid solubility, as a result they cannot easily cross lipid membrane barriers, such as the blood – brain barrier, renal tubular epithelium, gastrointestinal epithelium, or placenta
|
|
|
What does this mean?
|
Nondepolarizing neuromuscular blocking drugs do not produce central nervous system effects, renal tubular reabsorption is minimal, oral administration is ineffective, and maternal administration does not adversely affect the fetus
|
|
|
What factor also exerts a role in the pharmacokinetics of nondepolarizing blockers?
|
Redistribution
|
|
|
How can the variable pharmacologic responses of patients to nondepolarizing blockers be explained?
|
By differences in pharmacokinetics
|
|
|
How can pharmacokinetics of nondepolarizing muscle blockers be changed?
|
By many factors, such as hypovolemia, hypothermia, and the presence of hepatic or renal disease, or both
|
|
|
How are renal and hepatic elimination aided?
|
By access to a large fraction of the administered drug because of the high degree of ionization, which maintains high plasma concentrations of nondepolarizing neuromuscular blocking drugs and also prevents renal reabsorption of excreted drug
|
|
|
Which nondepolarizing neuromuscular blockers are markedly altered by renal disease, and why?
|
Renal disease only alters the pharmacokinetics of the long – acting nondepolarizing drugs, such as pancuronium. The intermediate – acting neuromuscular blockers are eliminated by the liver (rocuronium), by metabolism by plasma cholinesterase (mivacurium), by Hoffman elimination (atracurium or cisatracurium), or by a combination of these mechanisms
|
|
|
Age – related changes in the responsiveness of nondepolarizing neuromuscular blockers
|
There is no change in the pharmacodynamics of neuromuscular drugs, which is confirmed by similar dose – response curves in the elderly and young adults
|
|
|
What is shown to enhance the effects of neuromuscular blockade?
|
Neuromuscular blockade is enhanced by volatile anesthetics, which reflects a pharmacodynamic action as manifested by decreased plasma concentrations of nondepolarizing neuromuscular blocking drugs required to produce a given degree of neuromuscular blockade in the presence of volatile anesthetics
|
|
|
In addition to volatile anesthetics, what other drugs enhance neuromuscular blockade produced by nondepolarizing blockers?
|
Aminoglycoside antibiotics, local anesthetics, cardiac antiarrhythmic drugs, dantrolene, magnesium, lithium, and tamoxifen (an antiestrpgenic drug)
|
|
|
What drugs may diminish the effects of nondepolarizing muscle blockers?
|
Calcium, corticosteroids, anticonvulsant drugs (phenytoin),
|
|
|
What else can be associated with altered pharmacodynamic responses to nondepolarizing muscle blockers?
|
Some neuromuscular diseases, such as myasthenia gravis, Duchenne's muscular dystrophy
|
|
|
When does there appear to be resistance to the effects of nondepolarizing neuromuscular drugs?
|
Burn injuries appear to cause resistance as reflected by the need to establish higher plasma concentrations of drug to achieve the same pharmacologic effect as inpatients without a burn; there is also resistance in skeletal muscles affected by a cerebrovascular accident, perhaps reflecting proliferation of extra junctional receptors that respond to acetylcholine
|
|
|
Cardiovascular effects exerted by nondepolarizing blockers
|
Nondepolarizing drugs may exert minor cardiovascular effects through drug – induced release of histamine, effects on cardiac muscarinic receptors, or effects on nicotinic receptors at autonomic ganglia
|
|
|
Which drugs can cause hypotension?
|
Transient hypotension may occur with atracurium and mivacurium, but usually only at large doses, more than 0.4 and 0.15 mg/kilogram respectively
|
|
|
Factors that affect the magnitude of circulatory effects of neuromuscular drugs
|
Vary from patient to patient and depend on factors such as underlying autonomic nervous system activity, blood volume status, preoperative medication, drugs administered for maintenance of anesthesia, and current drug therapy
|
|
|
Neuromuscular drugs in critical care and critically ill patients
|
Acutely injured patients with multiple organ system failure, including sepsis, who require mechanical ventilation of the lungs for prolonged periods, usually more than six days, may manifest prolonged skeletal muscle weakness on recovery. This weakness is augmented by the skeletal muscle paralysis produced by nondepolarizing neuromuscular blocking drugs
|
|
|
Characteristics of this weakness
|
These patients exhibit moderate to severe quadraparesis with or without areflexia, but they usually retain normal sensory function; the time course of the weaknesses unpredictable and in some patients may progress or persist for weeks or months
|
|
|
What is the pathophysiology of this myopathy?
|
It is not well understood
|
|
|
Recommendations about neuromuscular blocking drugs in these patients
|
Should be given for two days or less and only after the use of analgesics, sedatives, and adjustments to ventilator settings have been maximally used
|
|
|
In addition to acute injury which patients may this occur in?
|
Asthmatics receiving corticosteroids
|
|
|
What is the important thing to remember about this myopathy?
|
It occurs autonomously, administration of neuromuscular blockers simply augments the severity of this condition
|
|
|
Use of succinylcholine in these patients
|
Should be used with extreme caution to facilitate endotracheal intubation in critically ill patients because of reports of cardiac arrest presumably caused by acute hyperkalemia
|
|
|
What is the dubious distinction neuromuscular blocking drugs carry during anesthesia?
|
They are responsible for triggering more than 50% of the anaphylactic and anaphylactic Lloyd reactions occurring during anesthesia
|
|
|
Rate of occurrence of anaphylactic reactions with neuromuscular blockers
|
Occur at a rate between one in 1000 and one in 25,000 anesthetics
|
|
|
Which neuromuscular blocker is the most common offender?
|
Succinylcholine
|
|
|
Even though it does not produce histamine release, which nondepolarizing neuromuscular blocker is been identified as producing an increased risk for allergic reactions?
|
Rocuronium was identified in France and Norway, with no confirmation from other countries
|
|
|
Recent follow-up study of neuromuscular blockers and anaphylaxis in Norway
|
A study of 83 cases of anaphylaxis during general anesthesia revealed that 77% of these were mediated by IGE and 93% were associated with neuromuscular blocking drugs, succinylcholine being the most common
|
|
|
Cross – sensitivity between all neuromuscular blockers
|
There may be cross sensitivity due to the presence of a common antigenic component, the quaternary ammonium group
|
|
|
Anaphylaxis after the first exposure to a neuromuscular blocker
|
May reflect sensitization from previous contact with cosmetics or soaps that also contain antigenic quaternary ammonium groups
|
|
|
long-acting neuromuscular blockers
|
pancuronium
|
|
|
pancuronium
|
-a bisquaternary aminosteroid nondepolarizing NMBD
|
|
|
pancuronium ED95
|
70 ug/kg
|
|
|
onset of action of pancuronium
|
3-5 minutes
|
|
|
duration of action of pancuronium
|
60-90 minutes
|
|
|
pancuronium elimination
|
-about 80% of a single dose is eliminated unchanged in the urine, thus renal failure causes plasma clearance of it to decrease by 30-50% resulting in prolonged duration of action
-10-40% undergoes hepatic deacetylation to inactive metabolites, except for 3-desacetylpancuronium, which is about 50% as potent as pancuronium |
|
|
cardiac effetcs of pancuronium
|
-gives a 10-15% increase in heart rate, MAP, and CO
|
|
|
HR increase due to pancuronium
|
-due to selective blockade of cardiac muscarinic receptors (an atropine-like effect), principally at the SA node
-indeed, the HR response still occurs in patients on B-adrenergic blockers |
|
|
what does the HR increase caused by pancuronium seem to depend most on?
|
-on the preexisting heart rate, with an inverse relationship, rather than the dose or rate of administration of the drug
|
|
|
which patients are most likely to have marked increases in HR caused by pancuronium?
|
-those with altered AV conduction, such as A-fib
|
|
|
what accounts for the modest increase in SBP caused by pancuronium?
|
the effect of the increased HR on CO in the setting of unchanged SVR
|
|
|
cardiac-stimulating effects of pancuronium
|
-could contribute to increased myocardial oxygen requirements, and lead to ischemia in patients with CAD
|
|
|
does pancuronium cause histamine release?
|
no
|
|
|
does it cause autonomic ganglion blockade?
|
no
|
|
|
intermediate acting nondepolarizing NMBDs?
|
include:
-vecuronium -rocuronium -atracurium -cisatracurium |
|
|
the intermediate-acting NMBDs in contrast to pancuronium
|
-have efficient clearance mechanisms that reduce the likelihood of significant cumulative effects with repeated injections or infusions
|
|
|
these drugs when compared to pancuronium
|
-have a similar onset of max blockade (with the exception of roc, which is more rapid and with large doses can parallel the onset of succ)
-have about 1/3 the duration of action -have a 30-50% more rapid rate of recovery -have minimal to absent cumulative effects -have minimal to absent CV effects |
|
|
vecuronium
|
-a monoquaternary aminosteroid nondepolarizing NMBD of intermediate action
|
|
|
ED95 of vec
|
50 ug/kg
|
|
|
onset of action of vec
|
3-5 minutes
|
|
|
duration of action of vec
|
20-35 minutes
|
|
|
clearance of vec
|
-undergoes both hepatic and renal excretion
|
|
|
vec metabolites
|
-are inactive, with the exception being 3-desacetylvecuronium, which has 50-70% the potency of parent compound
|
|
|
another important comparison of vec with pancuronium
|
-vec has increased lipid solubility, which facilitates biliary excretion
|
|
|
effect of renal failure on vec
|
-the effect on its duration of action is small, but repeated or large doses of vec can result in prolonged blockade
|
|
|
CV effects of vec
|
-its devoid of circulatory effect, even with rapid injection of doses exceeding 3x ED95
|
|
|
what does this emphasize?
|
vec lacks vagolytic effects and is not associated with histamine release
|
|
|
rocuronium
|
-a monoquaternary aminosteroid nondepolarizing NMBD
|
|
|
roc ED95
|
0.3 mg/kg
|
|
|
roc onset of action
|
1-2 minutes
|
|
|
roc duration of action
|
20-35 minutes
|
|
|
what does the lack of potency of roc compared to vec mean?
|
-its an important factor in determining the rapid onset of roc, think that when a larger number of molecules need to be given, as with the less potent roc, there are more molecules available to diffuse to the NMJ, causing a more rapid onset of action
|
|
|
roc onset of action compared to succ
|

-the onset to single twitch depression of a dose of 3-4x ED95 (1.2 mg/kg) resembles the onset of action of SCh 1 mg/kg
|
|
|
problem with a dose of roc 3-4x ED95
|
-this large dose needed to mimic the onset of SCh causes the duration of action of roc to resemble pancuronium
-but if 102x ED95 (0.6 mg/kg) is used, it does not produce intubating conditions as good as SCh, larger doses (1.2 mg/kg) are needed |
|
|
roc clearance
|
-is largely as unchanged drug in the bile
-deacetylation does not occur -about 30% is renally excreted, so renal failure patients can have a longer duration of action especially of large doses or infusions |
|
|
CV effects of roc
|
-arent any, even at large rapid doses
|
|
|
histamine release and roc
|
does not really occur
|
|
|
atracurium
|
-a bisquaternary benzylisoquinolinium nondepolarizing NMBD, which is a mixture of 10 steroisomers
|
|
|
atracurium ED95
|
0.2 mg/kg
|
|
|
atracurium onset of action
|
3-5 minutes
|
|
|
atracurium duration of action
|
20-35 minutes
|
|
|
clearance of atracurium
|
-by spontaneous nonenzymatic degredation at normal body temperature and pH (Hoffman elimination), AND by ester hydrolysis by nonspecific plasma esterases (so by both chemical and biological mechanisms)
|
|
|
metabolite of atracurium
|
-the major metabolite from both pathways is laudanosine; it is not active at the NMJ, but at high concentrations can cause CNS stimulation
|
|
|
atracurium clearance in patients with some important diseases
|
-the 2 routes of atracurium elimination which occur simultaneously are independent of hepatic, renal, or plasma cholinesterase function, so the duration of blockade is similar in normal patients and those with hepatic and renal disease, and those with atypical plasma cholinesterase (emphasizing that ester hydrolysis is unrelated to the plasma cholinesterase which hydrolyzes SCh and mivacurium)
|
|
|
what amount of atracurium elimination is accounted for by ester hydrolysis?
|
2/3
|
|
|
CV effects of atracurium
|
-a rapid dose of 2x ED95 usually does not cause any change in HR or BP, however one of 3x ED95 can give a transient increase in HR and decrease in BP
|
|
|
atracurium and histamine release
|
-causes a transient release of histamine which parallels the HR and BP changes
|
|
|
cisatracurium
|
-a benzylisoquinolinium nondepolarizing NMBD
|
|
|
cisatracurium ED95
|
50 ug/kg
|
|
|
onset of action of cisatracurium
|
3-5 minutes
|
|
|
duration of action of cisatracurium
|
20-35 minutes
|
|
|
cisatracurium structure
|
-is 1 of the 10 stereoisomers of atracurium isolated
|
|
|
cisatracurium clearance
|
-undergoes Hofman elimination to laudanosine, with plasma concentrations of this metabolite for lower after cisatracurium than with equivalent doses of atracurium, likely due to the greater potency causing less molecules to be administered and hence lower concentrations of metabolites
|
|
|
cisatracurium clearance compared to atracurium
|
in contrast to atracurium, does not have anything to do with nonspecific plasma esterases
|
|
|
cisatracurium in patient swith diseases
|
-similar to atracurium, clearance is independent of organs, so there is no change in duration of action in patients with renal or hepatic failure
|
|
|
CV effects of cisatracurium
|
-in contrast to atracurium, has no histamine-releasing effects, so causes no CV changes even with rapid administration of large doses
|
|
|
short acting nondepolarizing NMBD
|
mivacurium
|
|
|
mivacurium
|
-short acting benzylisoquinolinium nondepolarizing NMBDs
|
|
|
mivacurium ED95
|
80 ug/kg
|
|
|
onset of action of mivacurium
|
2-3 minutes
|
|
|
duration of action of mivacurium
|
-12-20 minutes
-so, is about 2x the duration of axction of SCh, and 30-40% of the intermediate acting NMBDs |
|
|
mivacurium structure and clearance
|
-is made up of 3 stereoisomers, 2 of which undergo hydrolysis by plasma cholinesterase at of rate about 88% that of SCh
-hydrolysis of these 2 stereoisomers is responsible for the short duration of action -as with SCh, duration of action is increased and hydrolysis decreased in patients with atypical plasma cholinesterase |
|
|
recovery from mivacurium blockade
|
-spontaneous recovery is rapid, so may not need any drug-induced antagonism if the effects are off already
-since neostigmine decreases plasma cholinesterases, it could theoretically actually slow the hydrolysis of mivacurium, but still moderate levels of blockade by mivacurium can be antagonized by neostigmine |
|
|
what explains this paradox?
|
the fact that neostigmine is actually a better antagonist of true cholinesterase than plasma cholinesterase
|
|
|
edrophonium effect on plasma cholinesterase
|
has little or no effect
|
|
|
CV effects of mivacurium
|
-are minimal at doses up to 2x ED95
-however, rapid administration at doses 3x ED95 can cause histamine release sufficient to transiently decrease BP and increase HR |
|
|
what is the most reliable method to monitor the effects of NMBDs?
|
-using a peripheral nerve stimulator to deliver an electrical stimulus and evaluating the mechanically evoked response
-this permits titration of the drug to the desired effect, and evaluation of recovery at the end of surgery, which is facilitated by giving anticholinesterase drugs (neostigmine) |
|
|
nerves most often stimulated
|
-the ulnar nerve at the wrist or the elbow, or the facial nerve on the lateral face
|
|
|
why is using the ulnar nerve popular?
|
because the adductor pollicis is solely innervated by the ulnar nerve
|
|
|
using the facial nerve
|
-observe the orbicularis oculi, which although it can be difficult to quantitate, may be necessary due to positioning
-also note that the response of the orbicularis oculi to facial nerve stimulation more closely approximates onset of block of the laryngeal muscles than adductor pollicis stimulation |
|
|
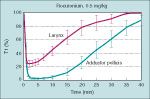
blockade onset of the laryngeal muscles compared with the adductor pollicis
|
-the onset of blockade after giving nondepolarizing NMBDs is more rapid but less intense at the laryngeal muscles (ie. vocal cords) than at the peripheral muscles (adductor pollicis), meaning the period of laryngeal muscle paralysis may be dissipating before a maximum effect is reached at the adductor pollicis
-in contrast, after SCh administration the onset of blcok at the laryngeal muscle compared to peipheral muscles is similar, thus adductor pollicis monitoring after SCh is more likely to parallel the effects of the drug on the laryngeal muscles |
|
|
types of mechanically evoked responses used to monitor NMBDs
|
include:
-single twitch response -train-of-four ratio -double burst suppression -tetanus -post-tetanic stimulation |
|
|
defining the depth of neuromuscular blockade and duration of drug effect
|
-the depth of neuromuscular block is defined as the percentage of a predetermined inhibition of twitch response from control height (ED95, the dose necessary to depress the twitch response 95%)
-duration of effect is the time from drug administration until the twitch response recovers to a percentage of control height |
|
|
duration to return to >25% control twitch height (minutes) for the common nondepolarizing NMBDs
|
pancuronium: 60-90 min
vecuronium: 20-35 min rocuronium: 20-35 min atracurium: 20-35 min cisatracurium: 20-35 min mivacurium: 12-20 min |
|
|
questions the response to peripheral nerve stimulation can be used to answer
|
1. is neuromuscular blockade adequate for surgery?
2. is the blockade excessive? 3. can the blockade be antagonized? |
|
|
characteristics of peripheral nerve stimulation that correlate with acceptable levels of skeletal muscle relaxation for intraabdominal surgery
|
-in the presense of adequate levels of volatile anesthetic, depression of twitch response greater than 90%, or elimination of 2-3 twitches of the TOF correlates
|
|
|
what if all twitches are abesnt on TOF?
|
should not give any more NMBD until some twitch is present
|
|
|
when is antagonism likely to be successful?
|
if some twitches on TOF are present
|
|
|
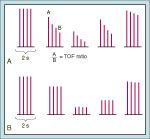
TOF
|
-giving 4 electrical stimuli at 2 Hz delivered every 0.5 sec
-based on the concept that ACh is depleted by successive stimulations -only 4 twitches are necessary, because subsequent stimulation fails to further alter the release of additional ACh |
|
|
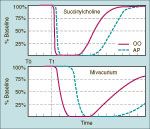
TOF after nondepolarizing versus depolarizing NMBDs
|
-after nondepolarizers, the 4th twitch will be decreased more than the 1st, allowing you to calculate a TOF ratio (fade)
-after SCh, the TOF ratio is 1.0, since all of the twitches are decreased by the same amount |
|
|
what does recovery of TOF ratio to >0.7 correlate to?
|
complete return to control height of a single twitch response
|
|
|
what does it mean if a TOF ratio is <0.3 in the presence of SCh?
|
phase 2 blockade
|
|
|
estimating the TOF ratio
|
-estimating the ratio is not reliable clinically by either manual or visual estimation
-difficulty is likely due to the fact that the 2 middle twitches interfere with comparison of the first and last twitch responses |
|
|
so what can be used?
|
double-burst stimulation
|
|
|
double-burst stimulation
|
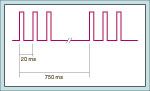
-2 bursts of 3 electrical stimulations separated by 750 msec, which is perceived by the observer as 2 separate twitches
-the ability to detect TOF ratio <0.3 is improved with this, but the ability to detect it >0.7 is still not ensured |
|
|
counting the number of TOF twitches
|
-this is much more likely to be achieved vs. trying to qunatify the TOF ratio
-for example, the 4th twitch can be seen when the 1st twitch is equal to 30-40% of control twitch height, corresponding to a TOF ratio of 0.35 |
|
|
reversal if 0 twitches
|
not recommended
|
|
|
reversal for <2 or 2 twitches
|
estimated TOF fade is ++++
anticholinesterase and dose: neostigmine 0.07 mg/kg IV anticholinergic and dose: glycopyrrolate 7 ug/kg OR atropine 15 ug/kg |
|
|
reversal for 3-4 twitches on TOF
|
estimated TOF fade: +++
anticholinesterase and dose: neostigmine 0.04 mg/kg anticholinergic and dose: glycopyrrolate 7 ug/kg OR atropine 15 ug/kg |
|
|
reversal for 4 twitches with an estimated TOF fade of ++
|
anticholinesterase and dose:
edrophonium 0.5 mg/kg anticholinergic and dose: atropine 7 ug/kg |
|
|
reversal for 4 twitches with an estimated TOF fade of 0
|
anticholinesterase:
edrophonium 0.25 mg/kg anticholinergic: atropine 7 ug/kg |
|
|
tetanus
|
continuous, or tetanic, electrical stimulation for 5 seconds at 50 Hz, which is an intense stimulus for the release of ACh at the NMJ
|
|
|
tetanus for nondepolarizing vs. depolarizing NMBDs
|

-in the presence of nondepolarizers, the response to tetanus is not sustained, or it fades
-whereas in the presence of SCh, the response is greatly decreased, but does not fade with phase 1 block |
|
|
at what TOF ratio is a sustained response to tetanus present?
|
>0.7
|
|
|
post-tetanic facilitation
|
-at the end of tetanus, there is an increase in immediately available stores of ACh such that the subsequent twitch responses are transiently enhanced
|
|
|
why might it be recommended to routinely use anticholinesterase drugs to antagonize the effects of nondepolarizing NMBDs?
|
-because even if all tests of the adequacy of neuromuscular function are normal, 50% of the receptors at the NMJ might still be occupied
|
|
|
uneqivocal evidence of adequate NMBD recovery
|
-5 seconds of head or leg lift
-tongue depressor test |
|
|
anticholinesterase use for reversing neuromuscular blockade
|
-they are usually given when spontaneous recovery from blockade is occurring so the effect of the pharmacologic antagonist adds to the rate of spontaneous recovery
|
|
|
mechanism of action of reversal agents
|
-accelerate the spontaneous rate of recovery by blocking the activity of acetylcholinesterase, thereby causing accumulation of ACh at nicotinic (NMJ) and muscarinic sites
-the increased ACh at the NMJ improves the chance that 2 ACh molecules will bind to the a-subunits of the nicotinic cholinergic receptors, which alters the balance of competition between ACh and NMBD in favor of ACh, restoring neuromuscular transmission |
|
|
additional effect of anticholinesterases
|
-may generate antidromic action potentials and repetitive firing of motor nerve endings (presynaptic effects)
|
|
|
structure of anticholinesterases and what it means
|
-they are quaternary ammoniums, which means their entrance into the CNS by crossing the BBB is greatly limited, allowing selective antagonism of peripheral nicotinic effects at the NMJ and other sites
-for example, peripheral cardiac muscarinic effects of these drugs include bradycardia, which can be attenuated by giving anticholinergics (glyco or atropine) |
|
|
factors that influence the success of NMBD antagonism
|
incldue:
-the intensity of the block at the time the antagonist is given -the choice of antagonist -the dose of antagonist -the rate of spontaneous recovery from the NMBD -the concentration of the inhlaed anesthetic |
|
|
most commonly used reversal agent
|
neostigmine
|
|
|
neostigmine reversal
|
-the greater the spontaneous recovery as judged by twitch monitoring, the more rapidly complete recovery will be by neostigmine
|
|
|
neostigmine dose
|
although the larger the dose given the more rapid the recovery will be, dose should be limited to 60-70 ug/kg
|
|
|
what other 2 things will affect the rate of recovery when giving neostigmine?
|
-the rate of recovery will be more rapid if the NMBD has a more rapid elimination (atracurium vs. pancuronium)
-the rate can also be hastened by reducing the concentration of volatile agent |
|
|
tests used to evaluate adequacy of reversal of blockade
|
-tidal volume
-TOF -vital capacity -sustained tetanus (50 Hz) -double burst supression -head lift -handgrips |
|
|
normal tidal volume
|
5 ml/kg
|
|
|
% of receptors occupied with a normal tidal volume
|
80%, means it is insensitive
|
|
|
% of receptros occupied with a TOF with no fade
|
70%, making this test somewhat uncomfortable
|
|
|
normal vital capacity
|
at least 20 ml/kg
|
|
|
% of receptors occupied with a normal vital capacity
|
70%; this required patient cooperation as well
|
|
|
% of receptors occupied with sustained tetanus at 50Hz with no fade
|
60%, and the test is uncomfortable for the patient
|
|
|
% of receptors occupied with double-burst suppression with no fade
|
60%, makes the patient uncomfortable
|
|
|
% of receptors occupied with 5 second head lift
|
50%, and it requires patient cooperation
|
|
|
% of receptors occupied with sustained 5 sec handgrips
|
50%, again requires cooperation
|
|
|
which of these tests indicates a TOF ratio >0.7?
|
-this is the ratio that is recommended, but it is difficult to establish clinically, so a sustained response to tetanus or head lift for 5-10 seconds usually indicates a TOF ratio >0.7
|
|
|
clinical implications of TOF ratio >0.7
|
-although it indicates the patient can sustain adequate ventilation, the pharyngeal musculatrue may still be weak so upper airway obstruction remains a risk
-also, diplopia, dysphagia, an increased risk of aspiration, and a decreased ventilatory response to hypoxia at TOF ratio >0.7 illustrate the value of the more sensitive clinical methods of assessing reversal like 5 sec sustained head lift, or masseter muscle strength (tongue depressor test) |
|
|
allowing spontaneous reversal of blockade without drugs
|
-is not recommended unless there is compelling clinical evidence that significant residual block doesnt persist
-it is not warranted to avoid drug-assisted antagonism with neostigmine for fear of inducing post-op nausea and vomiting |
|
|
questions to be answered when the initial response to a neuromuscular antagonist seems inadeuate before you give more reversal
|
1. has sufficient time elapsed for the anticholinesterase to antagonize the nondepolarizing NMBD (15-30 min)
2. is the degree of neuromuscular blockade too intense to be antagonized? 3. is acid base and electrolyte status normal? 4. is body temperature normal? 5. is the patient taking any drugs that could interfere with antagonism? 6. has clearance of the nondepolarizing NMBD from the plasma been decreased by renal or hepatic dysfunction or both? |
|
|
what is the new NMBD antagonist?
|
sugammadex
|
|
|
sugammadex
|
-y-cyclodextrin, drug under development which antagonizes steroidal NMBDs, especially roc, by encapsulating it, a mechanism totally different from neostigmine in that it has no action on any cholinesterase
-firther, no CV effects occur -if this is a successful drug, it would allow roc to be used for rapid tracheal intubation, and then be immediately reversed, reducing the need for SCh |

