![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
417 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
what are the steps in effective use of labs? |
•Perform a thorough history, physical exam-diagnosis will be evident 70+% of time • Develop a differential diagnosis (DDx) • Order appropriate disease- specific, targeted “screening” tests • Assess results for necessity of additional tests |
|
|
|
What is Osler’s rule regarding lab results? |
• If patient is under age 50, look for one etiology (cause) of all abnormal results |
|
|
|
When is general screening appropriate?
|
- targeted screening of high risk groups |
|
|
|
List and know biologic variability of test results with time.
|
• Circadian rhythm (24 hr. rhythm) - cortisol - pronounced diurnal variation • Rhythms less than or greater than 1 day • Timed tests - with relationship to meals --glucose: reference range given fasting --triglycerides - drugs: related to dose |
|
|
|
What are the effects on lab results of: Caffiene
|
- increased catecholamines, glucose - elevated lipids w/ chronic consumption |
|
|
|
What are the effects on lab results of: Alcohol
|
- casual use increases triglycerides - gamma glutamyl transferase / GGT (transpeptidase) --increased due to induction in moderate and heavy drinkers (w/ or w/o liver dis.) - no other consistent changes |
|
|
|
What are the effects on lab results of: Nicotine/tobacco smoke
|
• Increased: glucose, catecholamines, cortisol, free fatty acids • Increased WBC’s • Increased: CEA, carcinoembryonic antigen, a cancer marker • Increased carboxyhemoglobin- carbon monoxide content • Increased hemoglobin |
|
|
|
What changes in labs occur in the elder?
|
• Albumin, protein decrease starting mid-adult • Creatinine decreased : lower muscle mass • Muscle-related enzymes decreased • Lymphocytes reduced |
|
|
|
What changes in labs occur in young children?
|
• Lymphocytes must exceed 2500/μ ( adult normal is greater than 1500/ μ) • Alkaline phosphatase increased above adult reference range - enzyme product of osteoblasts |
|
|
|
How does exercise effect lab results?
|
• Strenuous exercise (marathon, etc.) - enzymes from muscles elevated - lactic acid increases • Well-trained athletes - lower mean cell hemoglobin (MCH), glucose, WBCs • Increased basal levels of muscle enzymes • Higher bilirubin; BUN |
|
|
|
What is the reference interval for lab tests?
|
• Gaussian distribution - occurs in tests with random fluctuations - usual interval is 2 SD from test mean; 95% --95% of persons w/o disease will have analyte result in this range • NonGaussian distribution is common - interval is set from 3rd to 97th percentile • Overlap between patients with disease and without disease • The larger the battery of tests ordered, the more likely one or more results will be abnormal (unreliable) |
|
|
|
Define: accuracy and contrast with precision. What is the “gold standard”?
|
• Accuracy: Reliability of the test method • Gold standard: recognized methodology against which new tests are compared • Precision: reproducibility of a result |
|
|
|
negative predictive value
|
TN/TN+FN |
|
|
|
positive predictive value
|
TP/(TP+FP) |
|
|
|
Sensitivity
|
Sensitivity % = (TP/(TP + FN))x100
|
|
|
|
specificity
|
Specficity % = (TN /(TN + FP))x100 |
|
|
|
what causes elevated BUN?
|
• Increased BUN ( with creatinine = Azotemia) - Almost all renal disease and - Poor renal perfusion -- dehydration, shock, heart failure, etc • Nonrenal causes of increased BUN - Catabolism - fever, burns, diabetes, intensive exercise - GI bleeding |
|
|
|
what causes elevated creatinine?
|
• Elevated-most renal disease – late ; poor renal perfusion, dehydration, etc • Insensitive but specific for renal impairment |
|
|
|
what causes elevated bilirubin?
|
• Jaundice= tissue accumulation of bilirubin - Prehepatic - hemolysis - Intrahepatic - liver disease: hepatitis, cirrhosis - Posthepatic - gallstone in bile duct |
|
|
|
what causes elevated alkaline phosphotase?
|
• Major sources: - liver -- canalicular (biliary) system - bone – osteoblasts - placenta - others include intestine (genetically variable); production by some cancers |
|
|
|
What causes elevated LD?
|
Glycolytic pathway enzyme • Widely distributed; elevated nonspecifically with a variety of tissue damage • Isoenzyme profile may be specific • Significant plasma elevation occurs with small amounts of tissue injury • High plasma levels associated with break-down of erythrocytes (hemolysis); platelets |
|
|
|
what casuses elevated AST?
|
- hepatocyte injury (“hepatitis”) - cardiac, skeletal muscle injury - hemolytic and megaloblastic anemias - renal and pulmonary infarction and other tissue necrosis |
|
|
|
what casuses elevated ALT? |
Liver is the major source • Lesser amounts released in renal infarction and very little from other tissues |
|
|
|
what is the chemistry profile of disease in muscle? |
• Muscle: - CK (creatine kinase) - AST - LD - Aldolase - Nonenzyme proteins: myoglobin |
|
|
|
what is the chemistry profile of disease in bone?
|
• Enzymes elevated in bone disease - Alkaline phosphatase |
|
|
|
what is the chemistry profile of renal disease?
|
BUN, late disease creatinine, AST, little ALT
|
|
|
|
what is the chemistry profile of hepatic disease?
|
• Synonym: Liver function tests or LFTs • Transaminases/aminotransferases - AST-aspartate aminotransferase (SGOT) - ALT- alanine aminotransferase (SGPT) • Alkaline phosphatase- ALP or Alk Phos • Gamma glutamyl transferase- GGT • LH or LDH -lactate dehydrogenase |
|
|
|
what is the chemistry profile of cardiac disease?
|
• Troponin I (and T) -- highly sensitive and specific -- currently the single, preferred test for lab confirmation of MI; elevated 1 wk. • Creatine kinase, CK - MB isoenzyme = “CK-MB” is cardiac specific; elevated before troponin • AST – nonspecific • LD – nonspecific • Myoglobin - nonspecific |
|
|
|
what is the chemistry profile of hemolytic anemia?
|
• LD markedly elevated • AST elevated • Indirect bilirubin increased • Haptoglobin decreased |
|
|
|
What does prealbumin assess?
|
• Sensitive indicator of - malnutrition - alterations in liver function • Short half-life of two days • Includes transthyretin (TTR): minor transport of thyroxine and vitamin A (complexes with retinol binding protein) |
|
|
|
What are causes of hypoalbuminemia? |
• Very common • Impaired synthesis : malnutrition, malabsorption, hepatic dysfunction • Increased loss - renal disease, esp. nephrotic syndrome - protein-losing gastroenteropathy - ascites |
|
|
|
What are the origin and correlates of changes in gamma globulins?
|
• Increased globulin is due to increase in gamma globulins, gammopathy • Types of gammopathies - Polyclonal gammopathy: due to prolonged infection or inflammation - Monoclonal gammopathy: neoplastic Decreased Gamma Globulins • Indicative of B-cell immunodeficiency, congenital or acquired • Risk for development of pyogenic infections |
|
|
|
List and know acute phase responses given in lecture.
|
Neutrophilia -Thrombocytosis (platelets increased) -Fibrinogen, other coagulation factors inc. -Alpha-2-macroglobulin increased -C-reactive protein increased -Haptoglobin increased -Albumin decreased -Erythrocyte sedimentation rate increased -Procalcitonin increased |
|
|
|
What are the clinical correlates of alpha-1 and alpha-2 globulins listed?
|
Alpha-1 globulins: Alpha-1 antitrypsin • α-1 antitrypsin is the major component of α-1 globulins - Protease inhibitor, PI, counters effects of leukocyte elastase • Acute phase reactant • Deficiency causes: - pulmonary emphysema - cirrhosis of the liver (misfolded protein is retained within hepatocyte) Alpha-2 globulins • Includes most of the “Acute phase reactants”: blood elements that are altered in response to acute inflammation • Alpha-2 macroglobulin: kinin inhibitor • Haptoglobin-carrier of free hemoglobin • C-reactive protein |
|
|
|
What are the uses of CRP?
|
• History: protein that reacts to C-substance of streptococci • General scavenger molecule • Acute phase reactant -rises fast in response to bacteria, necrosis: doubles q 6 hours -elevated in inflammatory response to autoimmunity; disease progression in RA, SLE • Correlates with risk of MI, stroke |
|
|
|
What is ESR and how is it used?
|
• Definition: length of fall of RBCs in a column of blood in a given time interval, mm/hr • Increased globulins, fibrinogen; decreased albumin favor accelerated sedimentation • Interpretation: - increased ESR is a nonspecific indicator of inflammation - used to follow rheumatoid arthritis, diagnose temporal arteritis and r/o inflammation |
|
|
|
What are the uses of procalcitonin?
|
• <0.15ng/dL, bacterial infection is unlikely • Between 0.15 and 2.0, localized infection cannot be excluded - elevation in autoimmune disease rarely exceeds 0.5 ng/dL • >2.0 highly favors severe bacterial infection: pneumonia, meningitis, sepsis correlates with progression to septic shock • Transient elevation also occurs: severe trauma, major surgery, severe burns |
|
|
|
Compare/contrast exudates with transudates as to causes and lab findings.
|
• Arise via increased vascular permeability • Exudates : high in protein, >3gm/dl; fibrin and other large molecules present - WBCs increased; LD (lactate dehydrogenase) often increased • Causes- -- infections/inflammations -- repair : angiogenesis -- malignancy : angiogenesis • Usually localized; generalized in septic shock • Low protein content; few cells; LD (lactate dehydrogenase) does not exceed plasma level • Causes: - Hydrostatic pressure increased: eg. heart failure - Osmotic pressure decreased: eg, liver disease, kidney disease, esp. nephrotic syndrome - Lymphatic obstruction - Primary retention of sodium (and water) --eg. renal disease; CHF / poor renal perfusion |
|
|
|
Table 4-1, Causes of edema: |
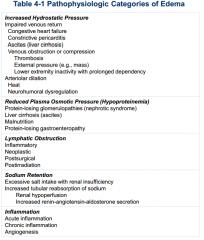
see picture |
|
|
|
What are some locations and what are the causes of local edema due to increased hydrostatic pressure?
|
• Edema due to impaired venous outflow. Examples, localized increased pressure: - dependent edema - deep venous thrombosis • Generalized (heart failure) |
|
|
|
What is the cause of hydrostatic pressure-related generalized edema?
|
Generalized: Congestive Heart Failure • Right ventricular dysfunction increases venous hydrostatic pressure • Plasma volume expands 2o to signals from poorly perfused kidneys |
|
|
|
Describe the pathogenesis of edema in heart failure (figure 4-2, Robbins) |
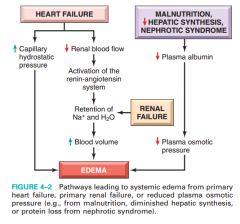
see picture |
|
|
|
mechanisms of edema in renal disease
|
Salt and Water Retention • Expands plasma volume - increases hydrostatic pressure - decreases colloid osmotic pressure by dilution • Retention most related to renal impairment - primary renal disease or secondary to renal hypoperfusion |
|
|
|
mechanisms of edema in liver failure
|
• Decreased protein/albumin production- |
|
|
|
mechanisms of edema in malnutrition
|
• Decreased protein/albumin production-
|
|
|
|
List causes of decreased colloid osmotic pressure.
|
• Increased protein loss: nephrotic syndrome, ascites, protein-losing enteropathy • Decreased protein/albumin production- - Severe liver disease (cirrhosis or other liver failure) - Malnutrition • Exacerbated by salt and water retention |
|
|
|
What is a frequent initial manifestation of edema related to decreased osmotic pressure?
|
Periorbital swelling
|
|
|
|
Define lymphedema. List causes. What is the description given to advanced lymphedema? What are complications of lymphedema?
|
• Primary: inherited or developmental defect of lymph vessels or nodes, eg Milroy dis. - onset may be at birth or delayed for yearsTrauma to lymphatic channels - removal of lymph nodes- cancer surgery - hip replacement • Radiation injury to lymphatics • Cancer metastatic to lymph nodes • Extrinsic pressure on lymphatics- tumor • Infections of lymphatics: “Elephantiasis” Wucheria bancrofti and other filariasis • Obesity • Peau d’orange in breast cancer: sign of late disease; poor prognosis • Complications of longstanding lymphedema - Secondary infection - Lymph vessel cancer (lymphangiosarcoma) |
|
|
|
What are the causes and morphology of pulmonary edema?
|
• Causes - left ventricular failure--common cause - renal failure - adult respiratory distress syndrome - pulmonary infections and other • Morphology, evolves from congestion - lungs 2-3x increase in weight - frothy, blood tinged fluid - Microscopic- see separate slides Clinical: hypoxia; risk for bacterial infection |
|
|
|
Define hyperemia and describe pathogenesis. What is the synonym and what are some causes?
|
• Hyperemia so known as erythema erythros = red; erythemia = flush - local increased volume of blood - active arteriole dilation red color due to increased oxygenated blood |
|
|
|
Describe passive congestion. What are some causes?
|
• Congestion - passive process due to impaired venous outflow from tissues - tissues are blue red (cyanosis); hypoxic - commonly associated with edema |
|
|
|
What is the morphology of chronic pulmonary congestion?
|
- Lung: rubbery, brown |
|
|
|
What is the morphology of chronic lower extremity edema? What complication may occur?
|
- Lower extremities: brown induration |
|
|
|
What is the cause and morphology of chronic hepatic congestion?
|
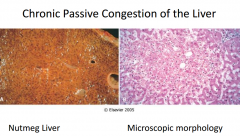
• Chronic passive congestion: Nutmeg liver fig 4-3 - Due to congestive heart failure - Centrilobular hemorrhagic necrosis - gross: red-brown depressed areas - may progress to “cardiac cirrhosis”- fibrosis • AST, ALT elevated due to necrosis of hepatocytes |
fig 4-3
|
|
|
What are causes of congestive splenomegaly?
|
- right heart failure - hepatic cirrhosis - portal vein occlusion |
|
|
|
Hematoma |
Mass of blood confined within an organ, tissue or internal space |
|
|
|
Contusion
|
• Contusion-bruise/ecchymosis: - Blunt force injury damages small blood vessels w/o disruption of continuity of tissue |
|
|
|
Ecchymosis
|
• Ecchymosis: large areas >1-2cm - changes over time from red-blue, to -- blue green: heme converted to bilirubin -- to gold-brown: hemosiderin |
|
|
|
Purpura
|
• Purpura: confluent petechiae. >3 mm - similar causes to above, plus vascular inflammation, trauma |
|
|
|
Hemarthrosis
|
bleeding into a joint |
|
|
|
Epistaxis
|
nosebleed |
|
|
|
Hemoptysis
|
coughing up blood |
|
|
|
Hematemesis
|
vomiting up blood |
|
|
|
Hematochezia
|
bright red blood in stool
|
|
|
|
Melena
|
black/digested blood in stool |
|
|
|
Hematuria
|
blood in urine |
|
|
|
Abnormal uterine bleeding (formerly menorrhagia, metromenorrhagia, etc)
|
excessive menstrual bleeding |
|
|
|
What are signs and symptoms of defects primary hemostasis?
|
• “ Superficial bleeds” • Epistaxis-nosebleeds • Bleeding from gums • Bleeding from/ into GI (gastrointestinal) tract and skin (typically multiple) - Petechiae (punctate hemorrhages) - Purpura: confluent petechiae - Ecchymosis: large areas >1-2cm • Bleeding from GU (genital urinary) tract |
|
|
|
Compare and contrast primary hemostasis deficiencies with signs and symptoms of coagulation factor deficiencies
|
• Primary hemostasis - Immediate response to vascular injury - Vascular wall- Endothelial cells and underlying connective tissue - Platelets • Secondary hemostasis - Blood proteins- procoagulants - Anticoagulants, fibrinolytic agents |
|
|
|
What are the prothrombotic effects of endothelium?
|
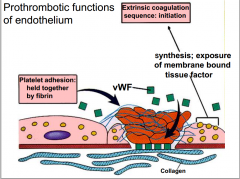
see picture |
|
|
|
Describe and know in order, the events involved in primary hemostasis (figure 4-4, A and B). List mediators involved in each step and actions of each. |
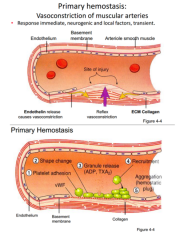
|
|
|
|
What are the hereditary platelet diseases? Be familiar with the clinical features (refer to signs and symptoms of diseases of primary hemostasis) |
- Glansmann thrombasthenia, hereditary defect of GpIIbIIIa Additional Acquired defects in platelets • Thrombocytopenia- decreased numbers. - Decreased production in bone marrow - Increased peripheral destruction: eg. autoimmune - ITP; HIV Sepsis • Dysfunctional platelets - Uremia (kidney failure) -Drug induced |
|
|
|
Clopidogrel (Plavex); Cangrelor
|
ADP receptor PY2 inhibitors (anti-platelet)
|
|
|
|
GpIIbIIIa inhibitors |
GpIIbIIIa inhibitors(antiplatelet)
|
|
|
|
Aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS)
|
- Block cyclo-oxygenase to prevent production of TxA2 (thromboxane) |
|
|
|
What are other acquired, nonpharmaceutical defects of platelets?
|
• Thrombocytopenia- decreased numbers. (details to be discussed in a later lecture) - Decreased production in bone marrow - Increased peripheral destruction: eg. autoimmune - ITP; HIV Sepsis • Dysfunctional platelets - Uremia (kidney failure) - DRUGS (antiplatelet) |
|
|
|
Review and recognize lab results likely to occur in defects of primary hemostasis?
|
• CBC including platelet count - reference range 150,000-350,000/μL (mm3) • Platelet function analyzer - measures time to form a platelet plug - analogous to bleeding time • Bleeding time: not used in most labs • Ristocetin agglutination test for vonWillebrand disease • Platelet aggregation studies |
|
|
|
Review coagulation (secondary hemostasis).
|
• Successive amplifying enzymatic reactions (“cascade”) terminating in activated thrombin • Thrombin proteolyzes fibrinogen to fibrin monomers. • Fibrin monomers polymerize into insoluble gel that encases platelets, other cells, to form definitive secondary hemostatic plug. • Factor XIII cross-links and stabilizes fibrin polymers. • Antithrombotic mechanisms limit extent |
|
|
|
Memorize vitamin K dependent factors.
|
-- procoagulants II, VII, IX, X -- anticoagulants proteins C and S • Coumadin/warfarin (“blood thinner”): -Vitamin K antagonist |
|
|
|
Prolonged PT only
|
Factor VII defects
|
|
|
|
Combined prolonged aPTT and PT
|
Medical conditions: anticoagulants, DIC, liver disease, vitamin K def., massive transfusion Rare: dysfibrinogenemia; factor X, V or II defect |
|
|
|
Prolonged aPTT only
|
Associated with bleeding: VIII, IX and XI defects Not assoc. with bleeding: XII, prekallikrein (PK), HWMK and lupus anticoagulant |
|
|
|
What does Prothrombin time (PT) measure?
|
• Time to clot measured ~ 12-14 seconds • Prolonged in: - extrinsic factor and common pathway factor deficiencies VII, X, V, II, I • Used to monitor coumadin therapy - INR: ratio of patient to control |
|
|
|
What doesPartial thromboplastin time , aPTT measure?
|
Time to clot formation is measured: usually < 40 seconds • Prolonged in - deficiencies of intrinsic system and common pathway factors: XII, XI, XI, VIII, X, V, II, I - circulating anticoagulants, eg. heparin • Monitors unfractionated heparin therapy (LMW heparin therapy requires no labs) |
|
|
|
What is the inheritance pattern of von Willebrand disease?
|
Autosomal Dominant |
|
|
|
What would thrombin time tests look like in Von Willebrand deficiency?
|
PT:unaffected aPTT: prolonged or unaffected bleed time: prolonged |
|
|
|
What is the inheritance pattern of Factor VIII deficiency?
|
X-linked
|
|
|
|
What thrombin times are seen in Factor VIII deficiency?
|
Hemophilia A PT: Normal aPTT:prolonged bleed time: normal |
|
|
|
What is the inheritance pattern of factor IX deficiency?
|
X-linked hemophilia b |
|
|
|
What thrombin times are seen in Factor IX deficiency?
|
hemophilia Bprolonged or normal aPTTNormal PT, and bleed times
|
|
|
|
Other than von Willebrand, factor VIII, and IX deficiencies; what is the inheritance pattern of most clotting factor deficiencies?
|
Autosomal Recessive
|
|
|
|
Defects with Prolonged aPTT only and bleeding
|
VIII, IX and XI defects
|
|
|
|
Defects with Prolonged aPTT only and NO bleeding
|
XII, prekallikrein (PK), HWMK and lupus anticoagulant |
|
|
|
Defects with only prolonged PT
|
Factor VII defects
|
|
|
|
defects with combined prolonged aPTT and PT
|
Medical conditions: anticoagulants, DIC, liver disease, vitamin K def., massive transfusion Rare: dysfibrinogenemia; factor X, V or II defect
|
|
|
|
What are the antithrombotic effects of endothelium?
|
- Physically inhibits platelet adhesion - PGI2 (prostacyclin) , NO, ADPase • Fibrinolytic - Source of tPA (tissue plasminogen activator) • Heparin-like molecule- cofactor for antithrombin • Thrombomodulin binding – inhibits thrombin and activates protein C • Tissue factor pathway inhibitor - Inactivates TF; factor VIIa - Protein S in a co-factor |
|
|
|
What is anttithrombin?
|
aka antithrombin 3, it inhibits the activity of thrombin and other serine proteases (IXa, Xa, XIa, XIIa), it is activated by when endolethial cells bind it with heparin-like molecule… (heparin activates this) |
|
|
|
What are proteins S and C?
|
Vitamin K dependent proteins that form a complex that inactivates factors Va and VIIIa. Process begins when thrombmodulin activates protein C. |
|
|
|
What is tissue factor pathway inhibitor?
|
secreted by endolethial cells, inactivate factors Xa and tissue factor-factor VIIa complexes. |
|
|
|
What condition is associated with deficiency or defect of a natural anticoagulant?
|
Thrombophilia; thrombosis |
|
|
|
Describe fibrinolysis
|
• Action: breakdown of fibrin to yield fibrin split products, FDPs (degradation products) and d-dimer - Inhibits fibrin polymerization; limits coagulation • Major activators - Factor XIIa dependent pathway - tPA (tissue plasminogen activator) - Urokinase-like plasminogen activator - Streptokinase, bacterial product |
|
|
|
what labs measure fibrinolysis?
|
fibrin degradation products(FDPs) --- d-dimer
|
|
|
|
what are the inhibitors of fibrinolysis?
|
fibrolysispicture |
|
|
|
Define thrombosis.
|
pathologic process-formation of a clot within intact vascular system |
|
|
|
What is Virchow’s triad? Refer to examples of thrombosis related to each of the three components of the triad.
|
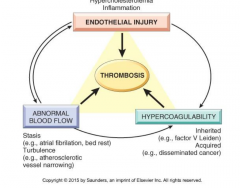
see figure 4-12 |
|
|
|
What do increased levels of factors VIII,IX,XI, or fibrinogen cause?
|
a common type of thrombophilia |
|
|
|
What does a G20210A mutation lead to?
|
Prothrombin mutation that leads to increased prothrombin levels; • Increased prothrombin drives more fibrinogen--> fibrin |
|
|
|
What are the rare thrombophilias?
|
Antithrombin III deficiency, Protein C deficiency, Protein S deficiency |
|
|
|
What are the features of Factor V Leiden?
|
ARG to GLU subsitution in amino acid 506 leads to resistance to activated Protein C; factor V Leiden |
|
|
|
What are features of hyperhomocysteinemia?
|
- Mutation in methyltetrahydrofolate reductase occurs in 3-5% of Caucasians, E. Asians --Elevated homocysteine inhibits: ATIII and thrombomodulin --- 2-5x risk for thromboembolism -- Increased risk for atherosclerosis - Homozygous deficiency of cystathione β- synthetase |
|
|
|
When would you suspect an inherited thrombophilia
|
Under age 50 Recurrent thrombosis unusual thrombosis sites (peripheral arteries) Thrombosis in pregnancy no apparent predispostion preceding thrombosis |
|
|
|
Risks for secondary/acquired hypercoagulable disorders.
|
Prolonged bed rest; immobilization •Myocardial infarction; atrial fibrillation • Tissue damage: surgery, esp orthopedic; fracture, burns, crush injury • Cancer: procoagulant tumor products • Prosthetic heart valves • Disseminated intravascular coagulation • Antiphospholipid antibody syndrome • Prior thrombosis at any time |
|
|
|
Which aquired hypercoagulable disorders confer the highest risk for thrombosis?
|

highriskthromb.png |
|
|
|
Describe HIT including pathogenesis and clinical features. |
Heparin-induced Thrombocytopenia, • Antibodies form to unfractionated heparin -Recognize complexes of heparin-platelet factor 4 - Binding activates platelets: formation of platelet rich thrombi -Thrombocytopenia due to platelet consumption - suspect when platelets drop by 50% • May (rarely ) occur with low molecular wt. heparin and fondaparinux (factor X inhibitor) |
|
|
|
Define antiphospholipid antibody syndrome, listing clinical associations
|
• May be primary: solitary autoimmune defect; or 2o to systemic lupus erythematosus • Fatal thromboembolic events occur in 7% • Recurrent venous or arterial thrombi, eg. - Arterial thrombosis: stroke, MI, bowel infarct - DVT with pulmonary emboli; pulmonary HTN - Renovascular hypertension - Renal microangiopathy renal failure |
|
|
|
Describe and recognize morphology of thrombi. Refer to figures 4-13 and similar examples.
|
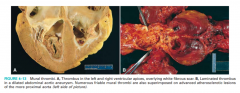
figure 4-13 |
|
|
|
What are the most common cause of arterial thrombosis and what are the consequences?
|
• Atherosclerosis is most common cause • Locations in order of frequency: • Coronary artery --> myocardial infarction (MI) • Cerebral artery thrombosis --> stroke • Femoral artery --> gangrene of the leg • Mural thrombus (w/in a chamber of heart) -> systemic emboli • Aneurysms also predispose to thrombosis |
|
|
|
What are the symptoms of DVT?
|
Asymptomatic in half • Found as consequence of pulmonary emboli • May result in edema, swelling of leg • Homan’s sign- pain in calf on dorsiflexion of foot |
|
|
|
Define terms that describe the fate of thrombi.
|
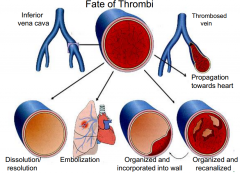
fate of thrombi picture |
|
|
|
What is the most common type of embolus?
|
Thrombi- cause of 99% of all emboli |
|
|
|
What are the causes and consequences of superficial saphenous vein thrombosis?
|
Associated with varicosities • Varicosities result in stasis; thrombosis • Thrombi produce local congestion, pain, swelling •Rarely embolize • Predispose to venous insufficiency; |
|
|
|
What are symptoms, morphology and consequences of pulmonary thromboembolism? Recognize figure 4-15 or equivalent.
|
Saddle embolus, large - Large embolus that occludes the bifurcation of the main pulmonary artery - Produces electrical mechanical dissociation (EKG activity with no pulse) • Massive simultaneous emboli involving 60% of pulmonary circulation - causes obstructive shock |
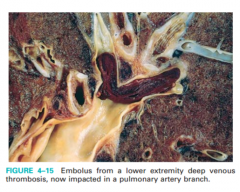
fig 4-15 |
|
|
Which site is most vulnerable for systemic emboli?
|
- Brain - 10% : stroke |
|
|
|
Which site is most common for systemic emboli?
|
- Lower extremities-75%: gangrene |
|
|
|
Traumatic fat embolism, figure 4-16
|
• Occurs with trauma, usually fracture of long bones (marrow) - shortness of breath followed by confusion, coma, 1-3 days after fracture; rash and thrombo-cytopenia; fatal in 10% |
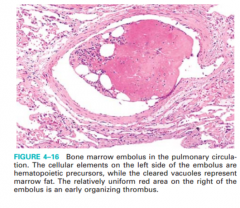
figure 4-16 |
|
|
Air embolism
|
• Clinical settings: exogenous air - Obstetric procedures-delivery/abortion contractions force air into injured vessels - pneumothorax -- Lung/chest wall injury opens large vein admitting air during inspiration - Clinically significant with >100cc air |
|
|
|
Amniotic fluid embolism, figure 4-17
|
• Infrequent complication of delivery, 1/40,000 • Mechanism: infusion of amniotic fluid into maternal circulation via tear in membranes and uterine wall • Morphology: Fetal squames, hair, vernix fat and meconium in pulmonary vessels • Clinical: shortness of breath, shock, followed by seizures, coma, DIC; fatal up to 80% |
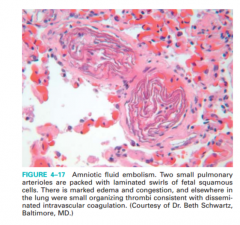
figure 4-17 |
|
|
Causese of infarction
|
Vascular lesions causing ischemia Thromboembolic disease causes 99% of infarctions Other: Vasospasm Expansion of atheroma Compression (tumor, tourniquet) Twisting of vessels: intestinal volvulus, testicular or ovarian torsion Edema, entrapment in hernia sac with vessel strangulation Vessel inflammation or rupture |
|
|
|
Red infarct
|
hemoragic infarct pic Venous occlusions: twisted ovaries, testes • Arterial occlusions in loose tissues (lungs) - blood collects in infarcted zone • Arterial occlusion in tissues with dual circulations: lungs, intestines • Occlusions in tissues previously congested • Occlusions where the blood flow is re-established |
|
|
|
White infarct
|
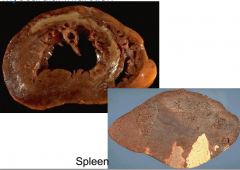
takes place in "solid" organs heart spleen |
|
|
|
bland infarct
|
no infection |
|
|
|
Septic infarct
|
presence of infection |
|
|
|
What conditions predispose for DIC?
|
Acute DIC • Occurs in obstetrical accidents, sepsis Chronic DIC • Presentation typically is venous thrombi - nonbacterial thrombotic endocarditis • Example: Trousseau syndrome - Migratory thrombophlebitis in a patient with pancreatic cancer |
|
|
|
Define DIC.
|
Thrombo-hemorrhagic disorder - consumptive coagulopathy: systemic activation of coagulation pathways leads to thrombi throughout the microcirculation • Secondary complication of variety of diseases |
|
|
|
What laboratory tests are used to assess patients for DIC?
|
--- platelets, coagulation factors are consumed --- fibrinolysis is activated |
|
|
|
Define shock (refer to consequences).
|
systemic hypoperfusion from reduction in cardiac output or reduced effective circulating blood volume |
|
|
|
Shock:What pH alteration occurs and what are its consequences?
|
Acidemia (increased serum lactic acid/ lactic acidosis)• Reversible cell injury that may progress to irreversible cell injury, death
|
|
|
|
What is the mechanism of cell injury in shock?
|
Hypoxia-->reduced ATP--> increased latic acid
|
|
|
|
signs and symptoms of shock.
|
• Related to increased catecholamines; vasoconstriction • Skin: pallor (pale color); cool, clammy (exception: initial response in septic shockis warm, dry skin) • Cyanosis, esp. around mouth; acrocyanosis (blue hands, feet) • Tachycardia with weak, rapid pulse • Tachypnea • Altered sensorium |
|
|
|
Cardiogenic shock
|
• “pump failure” primary myocardial impairment • Causes - Myocardial infarction -- common; in up to 15%; 70-90% mortality - Inflammation (myocarditis), intrinsic disease of myocardium(cardiomyopathy) - Arrhythmias, ventricular • Cardiac output decreased due to systolic anddiastolic dysfunction; arterial pressure reduced |
|
|
|
Obstructive shock
|
Often included in classifications of cardiogenic • Causes: - Massive pulmonary embolism - Cardiac tamponade (compression of heart due to fluid in pericardium) - Tension pneumothorax (air in pleural space that increases with each inspiration) - Mass lesions in chamber of heart • Cardiac output decreases because of decreased blood returning to left ventricle |
|
|
|
Septic shock
|
Common cause of death in hospitals: ~ 200,000/year; • Mortality: 20-50% • Causes: overwhelming microbial infections - Gram positive bacteria - Gram negative bacilli- endotoxic shock - Fungi • Other causes - superantigens/toxic shock syndrome - trauma, burns, pancreatitis |
|
|
|
Anaphalatic shock
|
• Type I hypersensitivity: IgE mediated • Histamine mediates vasodilation; increasedvascular permeability • Symptoms - dyspnea, tachypnea, swelling of airways - GI disturbances - tachycardia - altered sensorium • Common causes include peanut allergy; bee stings |
|
|
|
Hypovolemic/hemorrhagic shock
|
Occurs with acute loss of >20% of circulating blood or plasma volume • Cause: Hemorrhage - Trauma: wounds; fractures; perforations - Gastrointestinal bleeding - Aortic rupture • Cause: fluid loss –diarrhea, burns, dehydration • Cardiac output is decreased due to decreased blood volume (preload) |
|
|
|
With regard to septic shock, what are the pathogenesis (see also figure 4-20), clinical features unique from other shock, complications and sequelae?
|
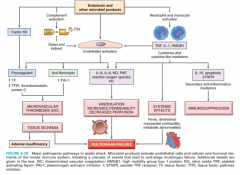
see 4-20 |
|
|
|
Morphology of shock
|
Kidneys: acute tubular necrosis/AKI • Lungs : diffuse alveolar damage- morphology of ARDS/shock lung • Liver: steatosis (fatty change); ischemic necrosis • Heart and CNS: ischemic necrosis • GI: hemorrhagic necrosis • Adrenal cortical lipid depletion • Disseminated intravascular coagulation:tissue ischemia; bleeding |
|
|
|
Nonprogressive phase of shock
|
eg blood loss that has ceased • Neurohumoral and renal mechanisms maintain cardiac output; blood pressure - fluid retention by kidneys - sympathetic stimulation-eg norepinephrine acts on α1 receptors on arteriole smooth muscle vasoconstriction (heart/brain spared) • Effects: - tachycardia - peripheral resistance increases |
|
|
|
Progressive shock
|
• Widespread tissue hypoxia yields lactic acid • Acidosis blunts the vasomotor response • Local arteriolar dilation; blood pooling - cardiac output reduced - renal insufficiency-injury develops - endothelial damage: endovascular clotting - increasing tissue hypoxia: organ failure - vascular permeability leads to edema |
|
|
|
Irreversible shock
|
• Lysosomal enzymes leak through damaged membranes- add to dysfunctions • Renal shutdown from acute kidney injury/acute tubular necrosis (ATN) • Myocardial contractility deteriorates • Ischemia of viscera often occurs: - GI flora enter circulation |
|
|
|
What is the most common cause of familial hypercholesterolemia?
|
• Heterozygotes - Plasma cholesterol increased from birth 2-3X • Homozygotes - Plasma cholesterol 5-6X normal (>500mg/dL |
|
|
|
what are the lab findings of hypercholestolemia?
|
Suspect familial hypercholesterolemia if: - Adults: Cholesterol > 350; LDL >270 - Children: Cholesterol > 270; LDL > 220 |
|
|
|
What is the defect, its clinical, including morphologic findings?
|
Heterozygotes - May exhibit cutaneous/tendinous xanthomas - Premature atherosclerosis develops in adults • Homozygotes - Skin and tendinous xanthomas in childhood - Coronary, cerebral and peripheral vascular atherosclerosis in childhood; adolescence |
|
|
|
What genetic defect is associated with hypertriglyceridemia?
|
- Hypertriglyceridemia various causes, including hepatic lipase deficiency |
|
|
|
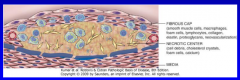
endothelial dysfunction
|
potentially reversible changes in functional state of endothelium in response to environmental stimuli |
|
|
|
Define endothelial activation
|
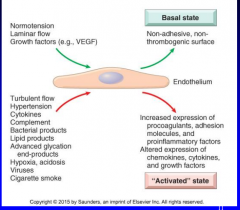
11-2chart |
|
|
|
Describe the three steps involved in healing damaged intima (Figure 11-3)
|
Migration of smooth muscle cells (SMC) from media to intima or SMC derived from circulating precursor cells –SMC proliferation (mitosis) –Elaboration of ECM by SMC |
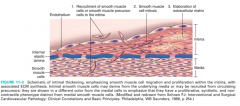
11-3 |
|
|
What are the two major hemodynamic variables regulating normal blood pressure? (Figure 11-4)
|
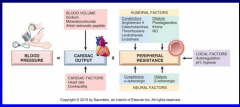
11-4 |
|
|
|
Define the terms essential, secondary, benign and malignant hypertension. Which is the most common? (Table 11-1) |
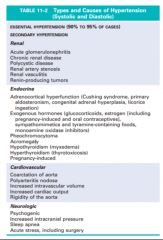
11-1 |
|
|
|
What are the mechanisms of essential hypertension?
|
• Inherited variations may depend on cumulative effects of polymorphisms in several genes that effect blood pressure • Environmental factors may initiate in genetically predisposed individual – Stress, obesity, smoking, physical inactivity • Vasoconstrictive influences • Reduced renal sodium excretion |
|
|
|
What form of hypertension is associated with hyperplastic arteriolosclerosis?
|
– Related to acute, severe elevations of blood pressure, characteristic of malignant hypertension – Onionskin, concentric, laminated thickening of walls of arterioles with progressive narrowing of lumen • May see necrotizing arteriolitis - deposits of fibrinoid and acute necrosis of vessel wall |
|
|
|
What form of hypertension is associated with hyaline arterilolsclerosis?
|
– Common in diabetes – Major characteristic of benign nephrosclerosis – Homogenous, pink, hyaline thickening of the walls of arterioles with loss of underlying structural detail and narrowing of the lumen |
|
|
|
What are the three patterns of aterioslcerosis? Which pattern is the most frequent and clinically important?
|
Atherosclerosis (dominant pattern) - intimal fibrous plaques with lipid rich core – Monckeberg’s medial calcific sclerosis - calcification of the media of muscular artery • Usually adult older than 50, no clinical significance – Arteriolosclerosis - proliferation or hyaline thickening of walls of small arteries and arterioles |
|
|
|
What is the lesion that characterizes atherosclerosis? Describe the major components. (Figure 11-7)
|
11-7 |
|
|
|
What are the constitutional risk factors for ischemic heart disease? (Table 11-2) What are the modifiable risk factors? (Table 11-2) |
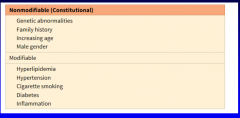
11-2 |
|
|
|
Describe the response to injury hypothesis (Figure 11-10)
|
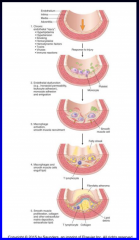
11-10 |
|
|
|
Using figure 11-11 as a model describe the major mechanisms of atherogenesis |
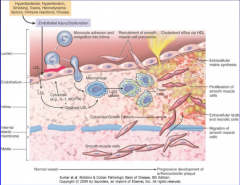
11-11 |
|
|
|
What is a fatty streak? At what age do they appear? Do all fatty streaks become fibrous plaques?
|
– Multiple yellow, flat spots, coalesce into elongated streaks – Lipid-filled foam cells – May appear before 1 yr, always present in older than 10 yrs – Not a clear cut relation to atheromas |
|
|
|
Recognize and describe the gross and microscopic findings (Figures 11-14, 11-15) Plaques |
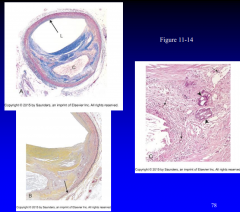
11-14 |
|
|
|
What are the three principle components of an atherosclerotic plaque? |
– Cells - SMC’s, macrophages, T lymphocytes – ECM – Lipids - intra and extracellula |
|
|
|
What clinically important complications/changes can happen to a plaque?
|
– Rupture/fissuring • Exposes thrombogenic plaque – Erosion/ulceration • Exposes thrombogenic subendothelial BM to blood – Hemorrhage into the atheroma |
|
|
|
Atherosclerotic stenosis
|
– Small vessels, plaque gradually occlude vessel lumen = ischemic injury – Critical stenosis • Chronic occlusion significantly limits flow, demand exceeds supply • Typically occurs at 70% fixed occlusion • Patients develop angina |
|
|
|
Acute plaque change (Figure 11-15) |
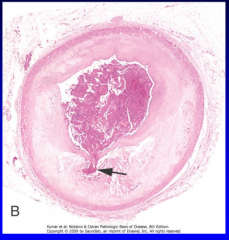
11-15 |
|
|
|
Thrombosis
|
Most serious = superimposed thrombus on partially stenotic plaque converts lesion to total occlusion – Pieces can embolize – Potent activator of growth-related signals in SMC’s |
|
|
|
Vasoconstriction
|
Adrenergic stimulation = increased BP, local vasoconstriction • Waking and rising = BP spikes = increased acute MI (6 am to 12 noon) |
|
|
|
How do atherosclerotic lesions cause disease?
|
-Gradual narrowing of lumina with ischemia of tissues – Site for thrombosis and embolization – Sudden occlusion of vessel by hemorrhage or superimposed thrombosis – Weakens vessel wall, leads to aneurysm or rupture |
|
|
|
What are the four major clinical consequences of atherosclerosis?
|
Myocardial infarction (heart attack) – Cerebral infarction (stroke) – Aortic aneurysms – Peripheral vascular disease (gangrene of legs) |
|
|
|
Be able to describe the pre-clinical and clinical phases of atherosclerosis (Figure 11-16)
|
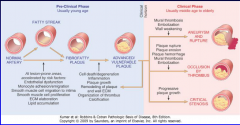
11-16 |
|
|
|
What is the difference between and true and a false aneurysm (Fig. 11-18)?
|
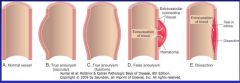
11-18 |
|
|
|
What factors with respect to the connective tissue in the vascular wall can lead to an aneurysm?
|
inherit weakness in connective tissue (marfans, etc) also inflamation-->increased mmp/decreased TIMP production scarring loss of smooth muscle |
|
|
|
Describe cystic medial degeneration. (Figure 11-19) |
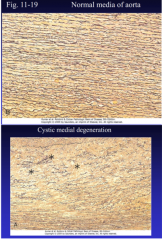
11-19 a b |
|
|
|
What are the two major disorders which predispose to aortic aneurysms?
|
• Atherosclerosis – abdominal aortic aneurysm (AAA) • Hypertension – aneurysms of ascending aorta |
|
|
|
What is a mycotic aneurysm?
|
- infection of a major artery that weakens wall • Originate from: – Septic embolus lodges in vessel – Extension of adjacent infection – Circulating organisms directly infecting |
|
|
|
Where do aneurysms associated with atherosclerosis most commonly occur?
|
abdominal aorta |
|
|
|
Who is more likely to get a AAA (age, sex, lifestyle)
|
smokers, men, after 50 |
|
|
|
What is a major cause of AAA?
|
atherosclerosis
|
|
|
|
Where are AAA’s usually located?
|
below renal arteries above bifurcation of aorta |
|
|
|
Describe the gross appearance of a AAA (Figure 11-20)
|
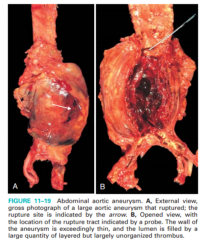
11-19actualy |
|
|
|
What are the five clinical consequences of AAA’s?
|
Rupture into peritoneal cavity, retroperitoneal tissue = potentially fatal hemorrhage – Impingement on adjacent structure – Occlusion of branch vessel = ischemia downstream – Embolism from atheroma or mural thrombus – Creation of abdominal mass – often palpably pulsating – Most are aymptomatic |
|
|
|
At or above what size are AAA’s at greatest risk for rupture?
|
>6cm >5cm managed surgically |
|
|
|
What are the clinical symptoms associated with a thoracic aortic aneurysm?
|
– Respiratory difficulties, encroaches on lungs, airways – Difficulty swallowing, compresses esophagus – Persistent cough, pressure on recurrent laryngeal nerve – Pain caused by erosion of bone – Cardiac disease – Rupture |
|
|
|
What disease is a TAA associated with?
|
hypertension
|
|
|
|
What two groups of patients do dissections occur in?
|
– Men 40 to 60 years old, 90% with hypertension – Patients with abnormality of connective tissue that affects aorta (Marfan syndrome) |
|
|
|
What is the major predisposing factor for dissections?
|
– Cystic medial degeneration |
|
|
|
What is the most frequent detectable microscopic lesion? (Fig. 11-21)
|
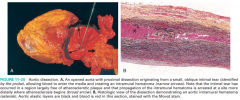
11-20 |
|
|
|
What is the DeBakey classification for dissections (Figure 11-22)?
|
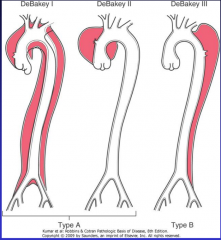
11-20 |
|
|
|
What are the classical clinical symptoms for a dissection?
|
– Sudden onset of excruciating pain, anterior chest, radiating to back, moving downward – Confused with acute MI
|
|
|
|
What is the most common cause of death with dissections?
|
rupture of dissection into any of three major body cavities (pericardial, pleural or peritoneal) |
|
|
|
What are the two most common mechanisms for vasculitis? Why is it important to distinguish between the two?
|
– Infectious pathogens - direct invasion – Immune-mediated inflammation • Need to distinguish for therapeutic reasons – Immunosuppressive therapy for immune |
|
|
|
What are the common clinical findings in vasculitis? |
Fever, myalgias, arthralgias, malaise –Tissue ischemia |
|
|
|
What are the three main mechanisms which initiate noninfectious vasculitis?
|
– Antineutrophil cytoplasmic antibodies • Patients serum reacts with cytoplasmic antigens in neutrophils – Anti-endothelial cell antibodies – Autoreactive T cells |
|
|
|
In what two ways can ANCA’s be useful clinically?
|
1. Certain types of vasculitis have specific association (eg c-ANCA=Polyangitis, P-ANCA=microscopic polyangitis, Churg-Strauss syndrome 2.Useful for diagnosis and following disease activity |
|
|
|
What is an ANCA? What are the two types?
|
(Antineutrophil Cytoplasmic antibodies) • Anti-myeloperoxidase (MPO-ANCA) – Old p-ANCA • Anti-proteinase-3 (PR3-ANCA) – Old c-ANCA |
|
|
|
Giant cell (temporal) arteritis affects what size vessels?What age group is it seen in?
|
medium arteries, typically in head, over age 50 |
|
|
|
What is the major morphologic finding of giant cell arteritis? (Fig. 11-24)
|
Segments of affected arteries develop nodular intimal thickenings with reduction of the lumen • Granulomatous inflammation, giant cells and fragmentation of internal elastic lamina |
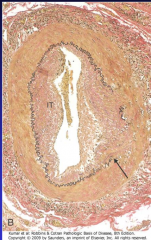
11-24 |
|
|
what are the symptoms of giant cell arteritis?
|
– Rare before age 50 – May have insidious presentation (fever, fatigue, weight loss) or sudden onset of headache, tenderness with swelling and redness, facial pain – Can often see polymyalgia rheumatica – Ocular symptoms serious (ophthalmic artery involved), occur in 50% of patients |
|
|
|
how is giant cell arteritis diaginosed definitively?
|
– Biopsy may be diagnostic but 1/3 are negative due to segmental nature of lesion, need 2-3 cm length of vessel – Negative biopsy doesn’t rule out disease |
|
|
|
what diseases are associated with giant cell arteritis?
|
polymyalgia rheumatica (PMR), which is characterized by sudden onset of pain and stiffness in muscles |
|
|
|
how is giant cell arteritis treated?
|
steroids
|
|
|
|
what size vessel does Takayasu arteritis affect?
|
medium and large arteries |
|
|
|
what is another name of Takaysu arteritis?
|
granulomatous vasculitis, pulseless disease
|
|
|
|
what are the two main symptoms of Takaysu arteritis?
|
Ocular disturbance and weakening of upper-extremity pulses |
|
|
|
what are the major morphological findings of Takaysu arteritis?
|
– Classically involves aortic arch, but 1/3 involve remainder of aorta and branches – ½ have pulmonary arteries involved – Irregular thickening of vessel wall with intimal hyperplasia – Aortic arch involvement = great vessels lumen decreased = pulseless disease – Mononuclear inflammation, granulomatous inflammation, then collagenous fibrosis |
|
|
|
what age group does Takaysu arteritis affect?
|
under age 50 |
|
|
|
what is the course of disease for Takaysu arteritis?
|
Course of disease variable, rapid progression or quiescent stage after 1or 2 yrs |
|
|
|
PAN affects what size vessels?
|
small or medium |
|
|
|
PAN affects renal and visceral vessels, what does it spare?
|
pulmonary
|
|
|
|
What are the morphologic features of PAN? (Fig. 11-26)
|
– Segmental transmural necrotizing inflammation – Three stages (all may coexist): • Acute - fibrinoid necrosis, neutrophils, eosinophils, mononuclear cells • Healing - transmural scarring, continuing necrosis • Healed - fibrotic thickening |
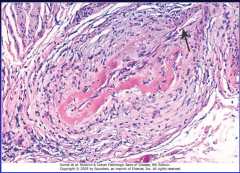
11-26 |
|
|
Do PAN patients get glomerulonephritis?
|
NOrenal artery involved, but not small renal vessls |
|
|
|
what age group does PAN affect?
|
30-49 young adults
|
|
|
|
what are common manifestations of PAN?
|
Fever of unknown origin, weight loss, hypertension, abdominal pain, melena, muscle aches and pains, peripheral neuritis |
|
|
|
What is the course of disease for PAN with treatment? Without?
|
Fatal if untreated, corticosteroids and cyclophosphamide result in 90% remission/cure |
|
|
|
How is PAN treated?
|
corticosteroids and cyclophamide |
|
|
|
What other vasculitis has the same characteristic lesion as Churg-Strauss syndrome?
|
PAN
|
|
|
|
What are the three features of Churg-Strauss syndrome?
|
Granulomas, infiltration of vessels and perivascular tissue with eosinophils |
|
|
|
What ANCA is Churg-Strauss syndrome associated with?
|
– P-ANCA present in 50% |
|
|
|
What is the other name for Kawasaki disease? What arteries does it involve?
|
Mucocutaneous lymph node syndrome involves corornary arteries |
|
|
|
What are the symptoms of mucocutaneous lymph node syndrome?
|
• Fever, conjunctival and oral erythema and blistering, edema of hands and feet, erythema of palms and soles and later desquamation • Cervical lymphadenopathy |
|
|
|
Patients with MLNS develop what complications? What are the morphologic features?
|
aneurysm that rupture or thrombose-->MI in children under 4 Transmural necrosis, inflammation like PAN but not as severe necrosis |
|
|
|
How does microscopic polyangiitis present?
|
palpable purpura involving skin or involvement of mucous membranes, lungs, brain, heart, GI tract, kidneys and muscle |
|
|
|
What size vessels are affected in microscopic polyangiitis?
|
Arterioles, capillaries, venules
|
|
|
|
Major clinical features of microscopic polyangitis?
|
hemoptysis, hematuria, proteinuria, arthralgias, bowel pain, muscle pain/weakness, hemorrhage |
|
|
|
How is a definitive diagnosis or microscopic polyangitis made?
|
•P-ANCA (MPO-ANCA’s) in 70% •Skin biopsy often diagnostic |
|
|
|
What two features distinguish microscopic polyangitis from PAN?
|
pulmonary involvement (pulmonary capillaritis) and glomerulonephritis |
|
|
|
Morphologic features of polyangitis(Fig. 11-27A)
|
Segmental fibrinoid necrosis or infiltration with neutrophils which fragment - leukocytoclasia |
|
|
|
What is the triad that characterizes Wegener Granulomatosis?
|
– Acute necrotizing granulomas of upper (ear, nose, sinus ,throat) and/or lower respiratory tract(lung) – Necrotizing or granulomatous vasculitis of small to medium size vessels mostly in lung and upper airway, can have other sites – Renal disease – focal necrotizing, often crescentic, glomerulonephritis |
|
|
|
What are the major morphological features of Wegener Granulomatosis?
|
Probable T-cell mediated hypersensitivity reaction - ? Inhaled infectious agent, environmental agent |
|
|
|
Wegener Granulomatosis affects what ages and sex?
|
Male >female, 40 yrs average |
|
|
|
What are the clinical features of Wegener Granulomatosis?
|
Pneumonitis with bilateral nodular and cavitary infiltrates, chronic sinusitis, ulceration of nasopharynx, evidence of renal disease |
|
|
|
What is the natural course of disease in Wegener Granulomatois?
|
– Untreated – 80% die within one year |
|
|
|
What ANCA is Wegener Granulomatosis associated with?
|
– C-ANCA (PR3-ANCA) in 95% - good marker for disease activity |
|
|
|
What is the other name for thromboangiitis obliterans?What group of people get it?
|
–Buerger Disease –Heavy cigarette smokers • Used to be predominantly male –Usually before age 35 |
|
|
|
What are the morphological features of thromboagiitis obliterans?
|
–Acute and chronic inflammation with luminal thrombosis –Microabscesses within thrombus, wall of granulomatous inflammation |
|
|
|
What can be done to prevent further attacks of thromboangiitis obliterans?
|
Stop smoking early in disease = relief from further attacks |
|
|
|
Clinical features? (Fig. 11-29)
|
– Paroxysmal pallor or cyanosis of digits of hands or feet – Involved digits show red, white, blue color changes, proximal to distal |
|
|
|
What is Raynaud phenomenon?
|
Exaggerated vasoconstriction of digital arteries and arterioles; Red, White, and Blue |
|
|
|
Who gets primary Raynaud phenomonen? What precipitates the attack?
|
– Exaggeration of normal central and local vasomotor responses to cold or emotion – Prevalence 3% to 5%, young female |
|
|
|
What is secondary Reynaud phenomonen?
|
– Vascular insufficiency of extremities, secondary to arterial narrowing from various processes • SLE, scleroderma, atherosclerosis and Buerger disease – Raynaud may be first manifestation of condition, rule out underlying cause |
|
|
|
What is the natural course of Raynaud phenomenon?
|
– Usually benign course
|
|
|
|
What are varicose veins?
|
Abnormally dilated, tortuous veins due to increased intraluminal pressure and loss of vessel wall support |
|
|
|
What veins typically become varicose?
|
superficial leg veins
|
|
|
|
what are predisposing factors for developing varicose veins?
|
Obese individuals and women (pregnancy) at risk • Familial tendency may be due to defective venous wall development |
|
|
|
what are the sequelae of varicose veins?
|
– Persistent extremity edema – Trophic skin changes • Stasis dermatitis • Ulcerations • Poorly healing wounds and infections may become chronic varicose ulcers • Vulnerable to injury – Embolism or other serious complications very RARE |
|
|
|
what are the clinical symptoms associated with varicose veins?
|
Venous stasis –Congestion –Edema –Pain –Thrombosis |
|
|
|
What veins account for majority of cases of thrombophlebitis and phlebothrombosis?
|
– Deep leg veins account for >90% of cases |
|
|
|
What is migratory thrombophlebitis' other name?
|
Trousseau sign
|
|
|
|
What factors predispose one for thrombophlebitis and phlebothrombosis?
|
• Prolonged immobilization resulting in decreased blood flow through veins, most important • Congestive heart failure, neoplasia, pregnancy, obesity • Postoperative state • Systemic hypercoagulability |
|
|
|
What are the local manifestations of thrombophlebitis and phlebothrombosis?
|
– Edema distal to occluded vein – Dusky cyanosis – Dilation of superficial veins – Heat, tenderness, redness, swelling, pain |
|
|
|
what are serious complications of thrombophlebitis and phlebothrombosis?
|
Pulmonary embolism serious complication of DVT • Pulmonary embolus may be first sign of DVT |
|
|
|
Clinical features superior vena caval syndrome ?
|
–Dusky cyanosis, –Marked dilation of veins of head, neck and arms |
|
|
|
The superior vena caval syndrome is cause by what?
|
Usually caused by neoplasms that compress or nvade superior vena cava • Primary bronchogenic carcinoma • Mediastinal lymphoma |
|
|
|
Clinical features inferior vena caval syndrome?
|
• Marked leg edema • Distention of superficial veins of lower abdomen • Massive proteinuria (renal veins involved) |
|
|
|
The inferior vena caval syndrome is caused by what?
|
– Neoplasms compress or penetrate walls of vena cava or thrombus may propagate upwards • Hepatocellular carcinoma and renal carcinoma have propensity to grow within lumens of veins with ultimate extension to vena cava, may cause obstruction |
|
|
|
When does systolic dysfunction occur?
|
•Ischemic injury, pressure/volume overload, dilated cardiomyopathy |
|
|
|
When does diastolic dysfunction occur?
|
•Massive LV hypertrophy, myocardial fibrosis, deposition of amyloid, constrictive pericarditis |
|
|
|
What three mechanisms does the cardiovascular system use to maintain pressure and perfusion in the face of damage or excess burden?
|
•Frank-Starling •Myocardial hypertrophy with/without cardiac chamber dilation •Activation of neurohumoral systems |
|
|
|
What two features characterize CHF?
|
•Diminished cardiac output - forward failure •Damming back of blood - backward failure |
|
|
|
What are the three most common underlying disease states for CHF? (Fig. 12-2)
|
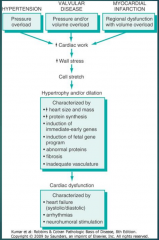
12-2 |
|
|
|
What are the two patterns of hypertrophy in the heart? (Fig. 12-2)
|
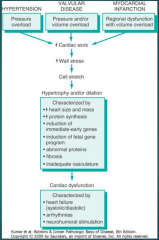
12-2 |
|
|
|
What four morphologic features are seen in the heart in CHF? (Fig. 12-1)
|
-Increased heart weight -Progressive wall thinning -Chamber dilatation -Microscopic changes of hypertrophy |
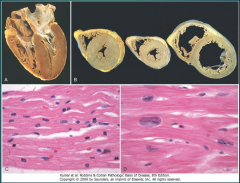
12-1 |
|
|
What are the four most common causes of left-sided heart failure?
|
•Ischemic heart disease •Hypertension •Aortic and mitral valvular disease •Nonischemic myocardial diseases |
|
|
|
What happens to the left atrium in left heart failure?
|
•Atrial enlargement occurs, leads to atrial fibrillation •May cause blood stasis with thrombus formation, especially in appendages •Leads to increased risk for embolic stroke |
|
|
|
What three changes occur in the lungs with left heart failure?
|
•Pressure in pulmonary veins increases, transmitted retrogradely leads to pulmonary congestion and edema •Perivascular and interstitial transudate •Edematous widening of alveolar septa |
|
|
|
What are heart failure cells?
|
- hemosiderin containing macrophages in alveoli |
|
|
|
What are the possible clinical manifestations of the lung changes?
|
•Dyspnea - breathlessness •Orthopnea - dyspnea on lying down relieved by sitting/standing •Paroxysmal nocturnal dyspnea - attacks of extreme dyspnea bordering on suffocation •Cough |
|
|
|
What is the most common cause of right heart failure?
|
Most commonly right sided heart failure is caused by left sided failure |
|
|
|
Pure right-sided heart failure occurs with what? What is it called in this case?
|
called cor pulmonale •RV pressure overload due to intrinsic disease of lungs or pulmonary vasculature |
|
|
|
What four changes occur in the liver in CHF?
|
•“Nutmeg” liver - chronic passive congestion, congested red centers of liver lobules surrounded by paler peripheral regions •Centrilobular necrosis •Central hemorrhagic necrosis - severe, rapidly developing failure leads to rupture of sinusoids •Cardiac sclerosis - long standing severe failure, central areas become fibrotic |
|
|
|
What happens to the spleen in CHF?
|
•Splenic congestion - enlarged, firm spleen, may weigh 500 to 600 gm (150 nl), fibrous thickening of sinusoidal walls = congestive splenomegaly |
|
|
|
What is ascites?
|
transudate in peritoneal cavity |
|
|
|
What is anasarca?
|
Massive and generalized peripheral edema |
|
|
|
What five categories of heart disease account for almost all cardiac mortality?
|
•Ischemic heart disease (80 to 90%) •Hypertensive heart disease and pulmonary hypertensive heart disease •Certain valvular disease •Congenital heart disease •Nonischemic (primary) myocardial disease |
|
|
|
What category of heart disease accounts for 80-90% of deaths?
|
ischemic heart disease |
|
|
|
In 90% or more of cases what is the underlying cause of myocardial ischemia?
|
90% of cases related to perfusion deficiency due to reduction in coronary blood flow (atherosclerosis of arteries) |
|
|
|
What is another name for IHD?
|
•Coronary artery disease (CAD) •Coronary heart disease (CHD) |
|
|
|
What four syndromes describe the clinical manifestations of IHD?
|
Angina pectoris (three variants) •Myocardial infarction •Chronic ischemic heart disease •Sudden cardiac death |
|
|
|
What two things have led to a decrease in death due to IHD?
|
•Prevention by changed lifestyle, decreases risk •Diagnostic and therapeutic advances |
|
|
|
What is acute plaque change?
|
abrupt plaque change followed by thrombosis |
|
|
|
What disruptions may occur in IHD?
|
•Hemorrhage •Rupture and fissuring • Erosion/ulceration |
|
|
|
What are the acute coronary syndromes?
|
•Unstable angina, •Acute MI •Sudden cardiac death |
|
|
|
Define angina
|
•Paroxysmal attacks of substernal or precordial chest discomfort caused by: • transient (15 seconds to 15 minutes) myocardial ischemia |
|
|
|
What are the 3 angina patterns called?
|
•Stable or typical angina •Prinzmetal’s or variant angina •Unstable or crescendo angina |
|
|
|
What are the symptoms and underlying cause for stable angina?
|
•Due to reduction of coronary perfusion to a critical level by chronic stenosing atherosclerosis •Relieved by rest and nitroglycerin and calcium channel blockers |
|
|
|
What are the symptoms and underlying cause for prinzmetal's angina?
|
•Pattern of episodic angina that occurs at rest •Due to coronary artery spasm •Elevation of ST segment on EKG •Responds to nitroglycerin and calcium channel blockers |
|
|
|
What are the symptoms and underlying cause for unstable angina?
|
•Pain occurs with progressively increasing frequency, precipitated with less effort, often occurs at rest, of prolonged duration •Induced by fissuring, ulceration or rupture of plaque with superimposed (partial) thrombus and possibly embolization •Often prodrome of MI = preinfarction angina |
|
|
|
What is an MI?
|
Heart Attack •Death of cardiac muscle resulting from ischemia |
|
|
|
What are the two types of MI and how do they differ?
|
•Transmural (more common) -Ischemic necrosis involves the full or nearly full thickness of ventricular wall in distribution of single coronary artery •Subendocardial -Ischemic necrosis limited to the inner on-third or at most one half of the ventricular wall, extends laterally beyond perfusion area of single artery |
|
|
|
Which sex is protected from MI until middle age?
|
Women have less MI until middle age |
|
|
|
What sequence of events occurs with an MI?
|
•Sudden change in morphology of plaque (hemorrhage, fissuring/rupture, ulceration/erosion) •Platelet exposed to collagen and plaque, leads to platelet activation, aggregation with formation of platelet mass - may give rise to emboli or occlusive thrombosis •Vasospasm due to mediators released by platelets •Activated platelets release factors predisposing to coagulation and favoring vasospasm •Often, within minutes thrombus evolves to become completely occlusive |
|
|
|
What other three possible events unrelated to atherosclerosis may occur causing an MI?
|
•Vasospam +/- atherosclerosis may cause deficit •Emboli from left mural thrombosis, vegetative endocarditis, paradoxic emboli from right •Unexplained – no atherosclerosis or thrombosis |
|
|
|
What seven things account for the location, size and morphologic features of an MI?
|
•Location, severity and rate of development of coronary occlusion •Size of vascular bed perfused by vessel •Duration of occlusion •Metabolic/oxygen needs of at risk myocardium •Extent of collaterals •Presence site and severity of coronary arterial spasm •Alterations in blood pressure, heart rate and cardiac rhythm |
|
|
|
How does a transmural infarct differ from a subendocardial infarct?
|
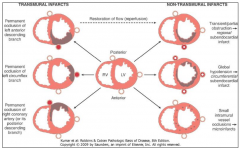
12-12 |
|
|
|
Describe the sequence of events grossly and microscopically for an MI (Table 12-5)
|
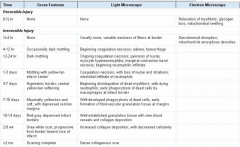
12-5 |
|
|
|
How may reperfusion to an infarct occur?
|
-Thrombolysis -Ballon Angioplasty -Coronary arterial bypass graft -Stent |
|
|
|
What pathologies may occur in an infarct with reperfusion?
|
•Reperfusion-induced arrhythmias •Myocardial hemorrhage with contraction bands •Irreversible cell damage due to the reperfusion itself •Microvascular injury •Prolonged ischemic dysfunction – Stunned myocardium |
|
|
|
What two laboratory measurements are useful to diagnose an acute MI? (Fig. 12-17)
|
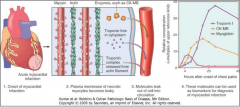
12-17 |
|
|
|
What four factors are associated with a poor prognosis after an MI?
|
• Diabetes, female, advanced age, previous MI |
|
|
|
What are the 11 possible complications following an MI? (Fig. 12-18)
|
– Cardiac arrhythmia's - conduction disturbances or myocardial irritability – Contractile dysfunction – Cardiac rupture syndromes - occur 3-7 days post MI – Infarct extension - new necrosis adjacent to old – Infarct expansion - stretching, thinning dilatation of infarct region – Ventricular aneurysm - late complication – Papillary muscle dysfunction – Mural thrombus – Progressive late heart failure – Right ventricular infarction – Pericarditis |
|
|
|
With regards to myocardial rupture which type is most common and when is it most likely to happen after an MI?
|
3-7 days after MI ventricular free wall most common and typically means death |
|
|
|
What happens with papillary muscle rupture? |
Papillary muscle rupture = acute mitral regurgitation |
|
|
|
What three things determine complications and prognosis after an MI? |
– Infarct size – Site – Transmural extent |
|
|
|
What age group is affected by Chronic Ischemic Heart disease?
|
elderly |
|
|
|
What is the clinical history of Chronic Ischemic Heart disease?
|
– Usually elderly, often prior MI, previous CABG – Insidious development of CHF as a result of ischemic damage - posinfarction cardiac decompensation |
|
|
|
what is the morphology of Chronic Ischemic Heart disease?
|
• Usually enlarged and heavy due to LV hypertrophy and dilation • Scars of previous infarcts, coronary vessel disease |
|
|
|
What is sudden cardiac death?
|
– Unexpected death from cardiac causes early (within 1 hour) after or without onset of symptoms |
|
|
|
What is the ultimate mechanism of death in sudden cardiac death?
|
lethal arrythmia |
|
|
|
What are the minimum criteria for diagnosing systemic hypertensive heart disease?
|
– Left ventricular hypertrophy (concentric) in absence of other causative pathology – History or pathologic evidence of hypertension |
|
|
|
What is the morphology of systemic hypertensive heart disease? Clinical ? (Fig. 12-20)
|
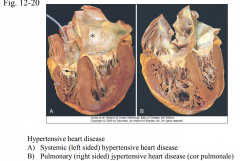
12-20 |
|
|
|
Pulmonary hypertensive disease is referred to as what?
|
cor pulmonale
|
|
|
|
What are the two types of cor pulmonale?
|
– Acute - RV dilation due to massive pulmonary embolism – Chronic - RV hypertrophy and later dilation secondary to prolonged pressure overload from obstruction of pulmonary arteries or compression of septal capillaries |
|
|
|
What are the four groups of disorders predisposing to cor pulmonale? (Table 12-7)
|
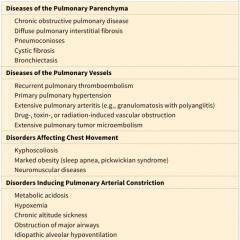
12-7 |
|
|
|
what is the gross morphology of cor pulmonale?
|
– Right ventricular enlargement secondary to pulmonary hypertension caused by disorders that effect lungs or pulmonary vasculature |
|
|
|
What is valvular stenosis? Insufficiency?
|
– Stenosis - failure of valve to open completely – Insufficiency (regurgitation) - failure of valve to close completely |
|
|
|
Two causes of regurgitation?
|
– valve incompetent due to dilation of ventricle or dilation of aortic or pulmonary artery
|
|
|
|
Mitral stenosis
|
rheumatic heart disease |
|
|
|
Mitral insufficiency
|
myxomatous degeneration (mitral valve prolapse) |
|
|
|
Aortic stenosis
|
calcification of anatomically normal and congenitally bicuspid aortic valves |
|
|
|
Aortic insufficiency
|
dilation of ascending aorta, related to hypertension and aging
|
|
|
|
What are the two types of calcific aortic stenosis?Which is more common?
|
senile calcific aortic stenosis (majority cases) and congenital bicuspid aortic valve |
|
|
|
What does the term senile calcific aortic stenosis refer to?
|
wear and tear due to age |
|
|
|
What is the hallmark lesion of calcific aortic stenosis? (Fig. 12-21)What is the gross morphology?
|
Heaped up nodular masses of calcium within sinuses of Valsalva, primarily at base, no commissure fusion
|
|
|
|
What is the anatomic change in the heart associated with mitral valve prolapse?(Fig. 12-22)
|
Myxomatous 'degeneration of the mitral valve – One or both valve leaflets are enlarged, redundant or floppy |
12-22
|
|
|
What are the serious complications of MVP?
|
– 3% develop complications • Infective endocarditis • Mitral insufficiency • Stroke of systemic infarct from thrombi that form on valve • Arrhythmias, sudden death uncommon • Most common cause for mitral valve surgery |
|
|
|
Who is at increased risk of having MVP?
|
Common condition, >/= 3% of population, young women |
|
|
|
Describe acute rheumatic fever of childhood - Jones criteria, labs (Fig. 12-23)
|
• RF – Jones Criteria –Major manifestations • Migratory polyarthritis of large joints • Carditis • Subcutaneous nodules • Erythema marginatum of skin • Sydenham’s chorea - neurologic disorder – --Minor • Fever • Arthralgias • Acute phase reactants • Diagnosis by Jones criteria – Evidence of streptococcal infection, presence of 2 major or 1 major and 2 minor |
|
|
|
What are the morphologic features of acute rheumatic pancarditis? Pathogenesis?
|
– Aschoff bodies • Collagen surrounded by lymphocytes and plump macrophages – Myocardium - Aschoff bodies – Pericardium - fibrinous pericarditis – Endocardium - fibrinoid necrosis with small vegetations (verrucae) |
|
|
|
What is the most important consequence of acute rheumatic carditis?
|
most important is develop ment of chronic rheumatic heart disease – Deforming fibrotic valve disease (mitral stenosis) • Severe, sometimes fatal, cardiac dysfunction decades later |
|
|
|
What are the features of chronic RHD?
|
Valvular stenosis and regurgitation (predominantly stenosis) – Most frequesnt cause of mitral stenosis (99%) – Mitral valve alone, 70% cases – Combined mitral, aortic 25% – Tricuspid less common, pulmonic very rare |
|
|
|
Define infective endocarditis (IE).
|
Microbial infection of heart valves or mural endocardium that leads to formation of vegetations composed of thrombotic debris and organisms |
|
|
|
What are the predisposing factors for subacute bacterial endocarditis? What organisim? What complications?
|
Clinical course of weeks/months • Previously damaged valves in half • Low virulence organisms are usual - Streptococcus viridans group (oral source) --most common cause: 60% of cases - Staphylococcus epidermidis infects prostheses - HACEK group, oral commensals - Streptococcus bovis (GI tract infections) - Enterococci (GU tract) |
|
|
|
What are the predisposing factors for acute bacterial endocarditis? What organisim? What complications?
|
-Heart valve usually normal prior to infection - Abrupt onset of fever and chills, lassitude - New murmur due to vegetations, valve destruction • Early complications of bacteremia and embolization of vegetations(metastatic infection) • Causes: virulent organisms - eg. Staphylococcus aureus in IV drug users |
|
|
|
What valves are most commonly affected by endocarditis?
|
-mitral and/or aortic are most common: source of systemic emboli - tricuspid in 50% of IV drug-use related IE : source of pulmonary emboli |
|
|
|
What are the clinical features of systemic septic embolization?
|
Janeway lesions |
|
|
|
What are usual cause and types of complications related to tricuspid endocarditis
|
Lung abscess: may be due to embolization from tricuspid vegetations |
|
|
|
Describe the cardiac morphology and cardiac complications of infective endocarditis.
|
• Vegetations on heart valves are bulky, friable (easily reduced to powder) • Localization of vegetations on valves - atrial side of AV valves - ventricular surface of semilunar valves • Valves involved: - mitral and/or aortic are most common: source of systemic emboli - tricuspid in 50% of IV drug-use related IE : source of pulmonary emboli |
|
|
|
What are the associations of NBTE?
|
Associated with debilitation: cancer; wasting (hypercoaguable states) |
|
|
|
Define Libman Sacks endocarditis and know associations.
|
LUPUS • Uncommon; due to current therapies • Small, sterile warty vegetations most commonly seen on undersurface of mitral/tricuspid valves • Micro: granular, fibrinous eosinophilic material with cellular debris/remnants - fibrinoid valvulitis of valve substance due complement activation; Fc-receptor cells • Thrombotic lesions may also be assoc. with antiphospholipid antibody syndrome |
|
|
|
What are the cardiac morphology and cause of carcinoid heart disease?
|
Cardiac lesion: Fibrous intimal thickening of right ventricle, pulmonic/tricuspid valves - acid mucopolysaccharide matrix deposited - tricuspid insufficiency is most common - pulmonic stenosis may occur - left side usually spared except when carcinoid tumor is in the lung or cardiac shunt present |
|
|
|
What other substances are associated with similar morphology to carcinoid heart disease?
|
Lesion on left due to drugs: ergots, phen-fen (fenfluramine) metabolized in lung to serotonin |
|
|
|
What ar the clinical feature of carcinoid syndrome?
|
- Episodic flushing, diarrhea, dermatitis andbronchoconstriction due to bioactive compounds such as serotonin released by carcinoid tumors |
|
|
|
What are the complications of artificial heart valves?
|
• Thromboembolic disease • Infective endocarditis - uncommon • Structural deterioration is the major cause of bioprosthesis failure • Nonstructural dysfunction - hemolytic anemia- shear forces - inadequate healing with paravalvular leak - exuberant healing with fibrosis |
|
|
|
How are bioprosthetic and mechanical type valves different? Need for anticoagulation?
|
• Mechanical - tilting disks; semicircular flaps - requires life long anticoagulation • Bioprosthetic- treated; largely nonviable - treated animal tissue: porcine aortic valve; bovine pericardium or human cadaver valve - graft rejection does not occur - prone to failure and requiring replacement • Serious complications occur in 60% w/in 10 yrs |
|
|
|
What is cardiomyopathy?
|
heterogenous group of diseases of myocardium…usually exhibit inappropriate ventricular hypertrophy or dilation …variety of causes…frequently genetic…confined to heart or part of systemic disease…often lead to cardiovascular death |
|
|
|
What causes hypertrophic cardiomyopathy?
|
- Hypertrophic: diastolic dysfunction
|
|
|
|
What causes dilated cardiomyopathy?
|
Dilated (DCM): systolic dysfunction
|
|
|
|
What causes restrictive cardiomyopathy?
|
Restrictive: diastolic dysfunction |
|
|
|
Is hypertrophic cardiomyopathy a systolic or diastolic dysfunction?
|
diastolic |
|
|
|
Is dilated cardiomyopathy a systolic or diastolic dysfunction?
|
systolic |
|
|
|
Is restrictive cardiomyopathy a systolic or diastolic dysfunction?
|
systolic |
|
|
|
what is the morphology of hypertrophic cardiomyopathy? Recognize 12-32; 12-34
|
• Heavy muscular hypercontracting heart developing in the absence of identifiable extrinsic stress • Incidence 1 /500; heterogenous; genetic • Left ventricular ejection fraction 50-80% (normal) • Mechanism of failure: impaired compliance; diastolic dysfunction; reduced filling • Intermittent ventricular outflow obstruction in 1/3 |
|
|
|
what is the morphology of dilated cardiomyopathy? Recognize 12-32; 12-34
|
Globular shaped regurgitation Hypertrophy fibrosis |
|
|
|
what is the morphology of restrictive cardiomyopathy? Recognize 12-32; 12-34
|
ventricles normal size or slightly enlarged and firm; ***biatrial dilation*** Micro: variable fibrosis or specific infiltrate |
|
|
|
what are the clinical findings of hypertrophic cardiomyopathy?
|
• Impaired diastolic filling of left ventricle: reduced size; poor compliance “ heart failure with preserved ejection fraction” • Intermittent left ventricular outflow obstruction • Reduced output; increase in pulmonary venous pressure--> exertional dyspnea • Harsh systolic ejection murmur • Myocardial ischemia with angina • Ventricular arrhythmias |
|
|
|
what are the clinical findings of dilated cardiomyopathy?
|
• Age, any: peak 20-50 • Progressive CHF- shortness of breath, etc • Half die in 2 yrs. - heart failure - arrhythmia; secondary mitral regurgitation - pulmonary/systemic emboli • Systolic dysfunction: ejection fraction < 25% in terminally ill patients ( normal ~60%) • Treatment: ventricular assist; transplantation |
|
|
|
what are the clinical findings of restrictive cardiomyopathy?
|
• Heart failure due to diastolic dysfunction • Ejection fraction near normal • Morphology on echocardiogram; radiography - Ventricles: may demonstrate mild wall thickening; may be normal **** Atria are dilated**** |
|
|
|
what are the causes of dilated cardiomyopathy?
|
•Idiopathic--cause unknown • Previous myocarditis - viral; Chagas’ disease • Alcohol abuse; other toxicity • Peripartum cardiomyopathy: pregnancy • Supraphysiologic stress • Genetic/familial cause |
|
|
|
What chemotherapies are associated with cardiomyopathy?
|
Doxorubicin (Adriamycin), Tyrosine kinase inhibitors Her-2 neu (trastuzumab; Herceptin) blocker |
|
|
|
What is the morphology of cocaine/other stimulant and reperfusion myocardial injury?
|
Morphology: focal myocyte necrosis; contraction bands, sparse inflammatory cell infiltrate (Contraction band necrosis also occurs in reperfusion injury) |
layla
|
|
|
What cardiac pathology is associated with iron overload?
|
Cardiomyopathy due to Iron Overload • Mechanism of injury - Iron interference with metal-dependent enzyme systems - Reactive oxygen species |
|
|
|
What cardiac pathology is associated with hyperthyroidism?
|
Cardiomyopathy due to Supraphysiologic Stress (similar morphology to cocaine induced cardiomyopathy) |
|
|
|
What are the features of senile cardiac amyloidosis?
|
--- amyloid type is transthyretin; accumulates with aging (nonmutant) and when mutated --- African Americans > Caucasians |
|
|
|
What are the causes of restrictive cardiomyopathy?
|
- Amyloidosis- protein accumulates between cells - Radiation fibrosis; metastatic tumor - Sarcoidosis (inflammatory disease) - Inborn errors of metabolism (Pompe, Fabry and glycogen storage dis. III., Hurler syndrome) |
|
|
|
List 3 uncommon, distinctive causes of restrictive cardiomyopathy.
|
• Endomyocardial fibrosis - children; young adults in Africa; mural thrombus • Loeffler endomyocarditis - fibrosis associated with eosinophilia - PDGFR activation from myeloproliferative disorder causes fibrosis; Tx imatinib • Endocardial fibroelastosis - childhood; congenital cardiac anomalies-1/3 - mutation mitochondrial gene; prenatal virus - thickened LV endocardium: insignificant to fatal |
|
|
|
Define myocarditis
|
***Inflammatory*** involvement of heart muscle characterized by a leukocyte infiltrate not secondary to ischemia; causes nonischemic degeneration of myocytes |
|
|
|
What are the most common causes of myocarditis? figure 12-35
|
- Infections are the most common cause - Immune-mediated reactions |
|
|
|
What type of myocarditis has the worst prognosis?
|
Chronic Chagas disease?? (Trypansoma cruzii)
|
|
|
|
What are complications of heart transplantation (page 572)?.
|
• Graft rejection • Complications of immunosuppression • Graft arteriopathy- coronary artery stenosis occurs at accelerated pace |
|
|
|
What alterations in laboratory tests are seen in heart failure?
|
• Creatinine, BUN increased - initially due to poor renal perfusion - prolonged failure --> permanent renal damage • Elevated AST, ALT from hepatic congestion/ nutmeg liver • N Terminal pro B natriuretic peptide; B natriuretic peptide: cardiac distension/ stretch • Galectin-3: marker for fibrosis; 40-50% of HF; predicts slower recovery; disease progression • ST2: interacts with IL-33; marker of inflammation,stretch. Correlates with poorer prognosis |
|
|
|
What are the clinical features of pericarditis?
|
• May be asymptomatic; present as enlarged heart on chest radiograph due to effusion • May resemble ischemic heart disease • Intense chest pain, accentuated on inspiration, relieved by sitting up • Pain may radiate to shoulder, trapezius ridge • Effusion - Slow accumulation tolerated - Rapid, as little as 200ml --cardiac tamponade |
|
|
|
What is the likely outcome of serous pericarditis? Morphology?
|
• Causes - Noninfectious inflammatory conditions - Viral infections eg, Coxsackie virus extension from myocarditis • Morphology: mild infiltrates of lymphs in epipericardial fat • Organization and adhesions uncommon |
|
|
|
What is the likely outcome of fibrinous pericarditis? Morphology?
|
Causes: • Acute myocardial infarction • Postinfarction (Dressler’s) syndrome: autoimmune reaction to MI several days to weeks later • Autoimmune disease- rheumatic fever; rheumatoid arthritis; SLE
• Morphology: “bread and butter” granular roughening • Friction rub may be heard on auscultation • Sequelae - Organization into adhesions is common |
|
|
|
What is the likely outcome of purulent pericarditis? Morphology?
|
• Due to bacterial infection • Examples: - Extension from pneumonia; - Ring abscess of endocarditis - Surgical introduction • Clinical findings same as fibrinous pericarditis • Sequelae - organization into adhesions; mediastinopericarditis or constrictive pericarditis |
|
|
|
What is the likely outcome of caseous pericarditis? Morphology?
|
Causes: Tuberculosis; some fungi • Outcome: Fibrocalcific, chronic constrictive pericarditis is likely |
|
|
|
What are the clinical features of cardiac tamponade?
|
• Triad of: - Hypotension (see obstructive shock) - Jugular venous distension - Muffled heart sounds • Pulsus paradoxus: systolic blood pressure during inspiration drops >10mmHg |
|
|
|
Describe adhesive mediatinopericarditis
|
• Healed pericarditis following suppurative or caseous pericarditis or cardiac surgery • Parietal pericardium adheres to heart and surrounding structures • Results in cardiac hypertrophy and dilatation |
|
|
|
What are causes, clinical features of constrictive pericarditis?
|
• Heart encased in fibrous or fibrocalcific scar • Diastolic expansion limited • Concretio cordis: extreme type- thick layer of calcification • Follows tuberculosis, suppurative infection or hemorrhage; may be idiopathic • Clinical: heart failure, quiet heart with reduced output; - cardiac hypertrophy/dilation cannot occur |
|
|
|
What are the morphologic features of 22q11 deletion? |
"Catch 22" --Cleft palate (other facial abnormalities) - Absent Thymus or hypoplastic thymus - Cardiac anomalies--outflow tract --truncus arteriosus; double outlet RV; tetralogy of Fallot and others - Hypoplasia of parathyroids; hypoparathyroidism--> hypocalcemia |
|
|
|
Describe the findings in right to left shunts
|
• “Cyanotic (blue) congenital heart disease” • Inadequate oxygenation of blood= cyanosis of skin/mucous membranes • Consequences - Paradoxical emboli - Hypertrophic pulmonary osteoarthropathy (clubbing of fingers) - Polycythemia- increased Hct/increased RBCs with risk of cerebral thrombosis |
|
|
|
Describe the findings in left to right shunts, including Eisenmenger syndrome
|
• Cyanosis initially absent • Increased pulmonary blood flow--> pulmonary hypertension - limits surgical correction of defects • Eisenmenger’s syndrome - “late cyanotic congenital heart disease” or cyanose tardive - L-to-R shunt becomes a R-to-L shunt due to pulmonary hypertension |
|
|
|
List causes of left to right shunts
|
• Ventricular septal defect VSD - most common heart defect • Atrial septal defect ASD • AV septal defect • Patent ductus arteriosus PDA
• (note “D” in name) |
|
|
|
What are complications of CHD in general?
|
• Cardiac hypertrophy and dilatation with heart failure • Infective endocarditis from jet stream injury • Retarded development/ failure to thrive • Increased complications of infectious diseases of childhood |
|
|
|
What type of atrial septal defect is most common?
|
1- Septum secundum 90% 2- Septum primum assoc w/ cleft mitral valve 3- Sinus venosus 4- Coronary sinus (rare) |
|
|
|
What are the features including complications of atrial septal defect (ASD)? |
Left-to-right shunt• May be isolated or associated with other defects • Presentation of isolated ASD- May be asymptomatic into adulthood- Large defects may present with neonatal CHF - Pulmonic valve murmur due to excessive blood flow- Pulmonary hypertension uncommon, <10% • Treatment: - Closure to prevent complications, eg. paradoxical emboli; infective endocarditis |
|
|
|
What are the frequency and significance of patent foramin ovale in adults?
|
• Fuses in 80% of neonates • Remaining 20% may re-open due to pulmonary hypertension - eg, multiple or recurrent PEMay lead to emboli formation |
|
|
|
What are the frequency, most common location and outcomes of VSD? |
• Left to right shunt • 20-30% isolated defect; other defects in rest • Defect types- Membranous septum- common- Muscular -- may close spontaneously -- may be multiple, “Swiss-cheese septum” |
|
|
|
What are the features of PDA? How is patency maintained; how might it be closed w/o surgery? Why maintain patency?
|
• Left-to-right shunt • Usually an isolated defect • Presentation- Machinery murmur- Normal cardiac function at birth • Maintain patency (for survival in some defects): administer prostaglandin E • To close: give indomethacin or ibuprofen (nonsteroidal anti-inflammatory drugs) • Closed becomes the ligamentum arteriosum |
|
|
|
What heart defect is associated with Down syndrome? What is the embryology?
|
Atrioventricular septal defect, AVSD; results from failure of fushion of endocardial cushions (neurocrest) |
|
|
|
What are the components of tetralogy of Fallot?
|
-VSD- Subpulmonary stenosis- Aorta overrides VSD; dextroposition-shifted rt.- Right ventricular hypertrophy |
|
|
|
What is the morphology of tetralogy of Fallot?
|
Boot-shaped heart--enlarged right ventricle |
|
|
|
What are the clinical features of tetralogy of Fallot?
|
• Degree of subpulmonary stenosis determines direction of shunting, outcomes |
|
|
|
What is the morphologic determinant of severity of disease for tetrology of Fallot?
|
Clinical Features of Tetralogy of Fallot dependent upon size of subpulmonic stenosis |
|
|
|
What genes are associated with tetrology of Fallot?
|
NOTCH pathway genes implicated |
|
|
|
Define transposition of great arteries
|
• Cyanotic at birth • Ventriculoarterial discordance - Aorta arises from right ventricle; is anterior and right of the pulmonic artery - Pulmonic artery arises from LV • Embryology: Abnormal formation of truncal and aortopulmonary “ spiral” septa |
|
|
|
Define persistent truncus arteriosus |
• Rt to L; early cyanosis • Single great artery overiding both ventricles; associated VSD • Embryology: Failure of separation of the truncus arteriosus into 2 vessels |
|
|
|
What syndrome is associated with truncus arteriosus?
|
Most common association: 22q11.2 deletion syndrome |
|
|
|
What is the association of tricuspid atresia?
|
hypoplasia of right ventricle |
|
|
|
Define TAPVC
|
Right-to-left shunt; cyanosis common • Pulmonary veins don’t enter L. atrium - Half feed into left innominate veins; coronary sinus • Patent foramen ovale or ASD always present • Volume and pressure hypertrophy of right heart • Left atrium is hypoplastic |
|
|
|
Define coarctation of the aorta
|
Obstructive anomaly; narrowing or constriction of aorta |
|
|
|
What are sex associations of coarctation of the aorta?
|
2x more common in males |
|
|
|
Describe adult form of coarctation of the aorta, including major, bolded associations.
|
- Valve defects: ***bicuspid aortic*** - ASD, VSD - Mitral regurgitation - ***Berry aneurysm***, circle of Willis |
|
|
|
What are the clinical features of coarctation of the aorta?
|
• Upper body hypertension • Hypotension of the legs - Cold/cyanotic legs - Claudication, intermittent--pain and limping brought on by walking • Characteristic x-ray finding: Rib notching • Clinical: Murmur throughout systole; Thrill • Cardiomegaly due to LV hypertrophy |
|
|
|
What are complications of coarctation of the aorta?
|
• Complications related to hypertension: ruptured cerebral aneurysm, stroke, heart failure • Infective endocarditis – of coarctation, bicuspid valve • Treatment: Surgical resection with anastamosis or prosthetic graft |
|
|
|
What is the most common type of valvular aortic stenosis?
|
Bicuspid aortic valve
|
|
|
|
What is the associated gene of the most common type of valvular aortic stenosis?
|
Bicuspid aortic valve, NOTCH2 |
|
|
|
What are complications of the most common type of valvular aortic stenosis?
|
Predisposes to premature calcific aortic stenosis; infective endocarditis |
|
|
|
Define hypoplastic left heart syndrome.
|
Severe stenosis or atresia of aortic valve • Underdeveloped left ventricle and aorta • Endocardial fibroelastosis • PDA necessary for life • Fatal first week of life if PDA closes |
|
|
|
List clinical features of Williams Syndrome
|
• Thickened ascending aorta; lumen of aorta is constricted = supravalvular stenosis • Multiple organ system disorder • Facial abnormalities: elfin, flat nose • Hypercalcemia in infancy (colic, vomiting, risk for metastatic calcifications) |
|
|
|
What is the genetic defect in Williams Syndrome? |
Deletion chromosome 7 -- includes elastin gene (ELN) and other genes assoc. with brain development |
|

