![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
117 Cards in this Set
- Front
- Back
|

|
|
|
|
|
|
Types of joints:
|
Synarthroses are immovable joints, amphiarthroses are slightly movable joints, and diarthroses are movable joints.
|
|
|
Components of Joints:
|
synovial membrane and fluid, periarticular ligaments and joint capsule, subchondral bone, and articular cartilage.
|
|
|
The synovial membrane layers:
|
intimal, subimtimal
|
|
|
Function of intimal layer:
|
responsible for the content of the synovial fluid
|
|
|
Composition of intimal layer:
|
1 to 4 cell layers thick and does not have a basement membrane. The cells of the intimal layers are synoviocytes
|
|
|
Functions of synoviocytes:
|
phagocytosis and protein secretion
|
|
|
types of synoviocytes:
|
A, B, C
|
|
|
function of type A synoviocytes:
|
phagocytosis or pinocytosis
|
|
|
function of type B synoviocytes:
|
protein secretion
|
|
|
function of type C synoviocytes:
|
both phagocytosis and protein secretion
|
|
|
Examples of intimal proteins:
|
hyaluronan, collagen, lubricin, pro-matrix metalloproteinases, IL, and eicosanoids
|
|
|
What is Steric exclusion?
|
process of separation of molecules by size, preventing large molecules from reaching the synovial fluid, and regulating fluid composistion
|
|
|
What molecules regulate steric exclusion?
|
Hyaluronan and lubricin
|
|
|
Composition of e subintimal layer:
|
fibrous, areolar, and fatty tissue. It contains blood vessels and nerves.
|
|
|
Function of the subintima:
|
allows smaller components of plasma to diffuse through the membrane from the vascular supply. Small molecules such as glucose, O2, CO2, and proteins less than 10kDa are diffusible to the synovial fluid, but larger molecules are not
|
|
|
Function of Periarticular ligaments and joint capsule:
|
stability to the joint
|
|
|
The function of the subchondral bone:
|
provide and contour and stability for the articular cartilage
|
|
|
How does subchondral bone differ from cortical bone?
|
arrangement of the haversian systems. In normal diaphyseal cortical bone, haversian systems are arranged parallel to the long axis of the bone but in subchondral bone the haversian systems are arrange parallel to the surface of the joint
|
|
|
What is the Nutrient supply to articular cartilage?
|
synovial fluid nutritional solutes diffuse through the cartilage matrix to the chondrocytes
|
|
|
Components of articular cartilage:
|
Chondrocytes account for 1-12% of the cartilage volume, with the remaining volume from the extracellular matrix
|
|
|
Composition of articular cartilage extracellular matrix:
|
collagen, proteoglycans and water
|
|
|
What portion of the ECM is water?
|
70%
|
|
|
Function of articular cartilage Collagen:
|
provide framework in which all the other components of the articular cartilage are confined and counteracts tensile forces on the joint surface
|
|
|
Layers of articular cartilage:
|
superficial, intermediate, deep, calcified
|
|
|
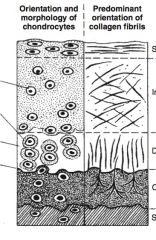
Appearance of chondrocytes in superficial layer:
|
most aabundant, aligned parallel to long axis of joint surface
|
|
|
Appearance and size of collagen in superficial layer:
|
small diameter collagen fibrils are aligned parallel to the long axis of the joint surface
|
|
|
What is the lamina splendens?
|
acellular layer of collagen in the superficial layer
|
|
|
Appearance of chondrocytes in intermediate layer:
|
oval to rounded chondrocytes
|
|
|
Appearance and size of collagen in intermediate layer:
|
larger diameter collagen fibrils are arranged in a random pattern
|
|
|
Appearance of chondrocytes in deep layer:
|
largest size of chondrocytes
|
|
|
Appearance and size of collagen in deep layer:
|
largest diameter collagen fibrils which are oriented perpendicular to the long axis of the joint surface
|
|
|
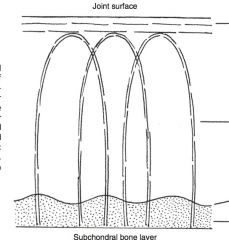
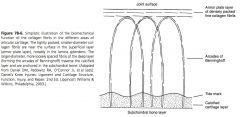
Whatis the e calcified zone?
|
mineralized cells and matrix
|
|
|
What is the tide mark?
|
junction of non-calcified and calcified cartilage.
|
|
|
Predominate type of collagen in articular cartilage?
|
type 2 collagen
|
|
|
How does type 2 collagen differs from type 1?
|
number of hydroxylysine molecules and the amount of glycosylation. The triple helical structure of type 2 collagen is composed of 3 identical molecules
|
|
|
Function of Type 9 collagen:
|
provides a covalent interface between type 2 fibrils
|
|
|
Function of Type 11 collagen:
|
found within the type 2 helix as a core filament onto which the type 2 molecules are deposited
|
|
|
Predominate proteoglycan in articular cartilage:
|
aggrecan
|
|
|
Function of aggrecan:
|
provides resistance to compressive forces
|
|
|
Composition of aggrecan:
|
core protein backbone and glycosaminoglycans that radiate from the core protein backbone
|
|
|
What are the main GAG that radiate form the core protein backbone of aggrecan?
|
Chondroitin 4 sulfate, chondroitin 6 sulfate and keratin sulfate
|
|
|
Function of GAGs in aggrecan:
|
repel each other and attract water, which creates a positive pressure
|
|
|
Domains of core protein of aggrecan:
|
G1, G2, and G3
|
|
|
What is the GI domain on the aggrecan core protein?
|
where the core protein interacts with hyaluronan
|
|
|
Compomposition of Hyaluronan:
|
forms a backbone to which 100s of aggrecan monomers are arranged
|
|
|
What is the G2 domain on the aggrean core protein?
|
adjacent to a keratin sulfate rich region and the target of enzymes that breakdown aggrecan
|
|
|
How do Chondrocytes respond to mechanical stress?
|
Low levels of mechanical stress favor catabolic processes but at normal physiologic levels of activity, anabolic processes are favored, resulting in a balance of metabolism. In superphysiologic mechanical stress, the anabolic pathways are overwhelmed by catabolic processes
|
|
|
What do Joint mechanics depend on?
|
kinematics, kinetics, and joint lubrication
|
|
|
What is Kinematics?
|
study of the motion of the articulating surfaces in relation to each other.
|
|
|
types of kinematic joint motions:
|
translation, rolling, sliding
|
|
|
what is Translational motion?
|
movement without rotation, such as 2 surfaces moving past each other
|
|
|
what is rolling motion?
|
the center of rotation is the point of contact between 2 surfaces and the distance of the surface contact is the same for both surfaces
|
|
|
what is sliding motion?
|
the point of contact is not the center of rotation and the contact area between the 2 surfaces is not equal
|
|
|
what is kinetics?
|
relationship of the forces that are created during motion of the joint and the load created across the surface of the joint
|
|
|
what does kinetics accounts for?
|
muscular, body weight, and contact forces acting on the joint in any given load bearing situation
|
|
|
function of boundary lubrication?
|
prevents adhesion and abrasion of 2 surfaces and does not depend on the physical properties of the lubricant or the surfaces
|
|
|
main boundary lubricants in the joint:
|
Hyaluronan and lubricin
|
|
|
What is created with Fluid-film mechanisms?
|
a wedge of fluid between 2 surfaces to provide low friction environment at physiologic loads
|
|
|
Possible models for fluid-film mechanisms:
|
are squeeze film, hydrodynamic, and elastohydrosynamic
|
|
|
What is Elastohydrodynamic fluid-film mechanics:
|
lubrication of the cartilage occurs because aggrecan attracting water to the articular surface. When the joint is loaded, water is squeezed out from the articular surface and interposed between the 2 surfaces
|
|
|
Why does Osteoarthropathy develop?
|
alteration of articular cartilage metabolism and articular cartilage matrix depletion
|
|
|
When does Alteration of articular cartilage metabolism occur?
|
when catabolic metabolism predominates, leading to tissue failure
|
|
|
How can catabolic pathwaysoverwhelm joint metabolism?
|
by being exposed to abnormal mechanical loads or a metabolic tissue failure
|
|
|
When do Abnormal mechanical loads occur?
|
from instability that overwhelms the normal reparative processes
|
|
|
What is involved in Matrix depletion?
|
synovial membrane and joint capsule, chondrocytes, matrix metalloproteinases, and cytokines
|
|
|
Histologic abnormalities seen in the synovial membrane during disease:
|
edema, hyperplasia of the intimal layer, increased vascularity, inflammatory cell infiltration and subintimal fibrosis
|
|
|
What does Articular cartilage degeneration results from?
|
loss of aggrecan and type 2 collagen, increased water content, increased surface fibrillation
|
|
|
Source of MMP?
|
synoviocytes and chondrocytes
|
|
|
Function of MMP?
|
collectively can degrade all portions of articular cartilage
|
|
|
MMPs involved in induction of OA:
|
MMP-1, MMP-8, MMP-13, which are all collagenases, MMP-3 is a stromolysin, and MMP-2 and MMP-9 are gelatinases
|
|
|
What breaks down aggrecan?
|
A structural and functional relative of the MMPs are the ADAM enzymes which is “a disintegrin and a metalloproteinase”
|
|
|
Function of MMP-1:
|
breaksdown type 2 & 10 collagen by breaking the each of the chains in the triple helix at a specific AA sequence
|
|
|
What upregulates MMP-13?
|
IL-1
|
|
|
Examples of catabolic cytokines:
|
IL-1, TNFa
|
|
|
Source of IL-1, TNFa:
|
chondrocytes and synoviocytes
|
|
|
Function of IL-1:
|
upregulation of MMP-13, upregulates production of NO and PGE2, inhibits synthesis of aggrecan and type 2 collagen, and inhibits production of natural anti-arthritic molecules such as TIMP and IL-1Ra
|
|
|
Function of NO:
|
degrade hyaluronan and collagen
|
|
|
Examples of cytokines that regulate pro-inflammatory cytokines:
|
IL-4, IL-10, IL-13
|
|
|
How do IL-4, IL-10, IL-13 regulate pro-inflammatory cytokines?
|
promoting synthesis of TIMP and IL-1Ra and inhibit the synthesis of IL-1
|
|
|
Function of TIMP (tissue inhibitor of metalloproteinases):
|
inhibit MMPs by binding on a one-to-one basis, forming an inactive complex
|
|
|
What TIMP are involved in inhibiting joint related MMPs?
|
TIMP-1, -2, and -3
|
|
|
Source of TIMPs:
|
secreted by chondrocytes and synoviocytes
|
|
|
major anabolic cytokines:
|
IGF and TGF
|
|
|
how do Sensory nerves produce pain from joint disease?
|
by responding to mechanical stimuli or to chemical mediators
|
|
|
examples of Chemical mediators:
|
kinins or neuropeptides like substance P
|
|
|
how do chemical mediators produce pain from joint disease?
|
affect pain fibers directly
|
|
|
what role do PGE2 and IL-1 have on joint pain?
|
sensitize pain fibers to be more reactive after mechanical stimulation
|
|
|
Why does joint fluid viscosity change?
|
decrease in hyaluronan or depolymerization of hyaluronan
|
|
|
What do Intrinsic repair mechanisms rely on?
|
chondrocytes to divide and repair the tissue
|
|
|
How are small articular cartilage defects repaired?
|
Matrix flow involves chondrocytes and matrix filling a defect from the periphery of the defect toward the center
|
|
|
What do extrinsic repair mechanisms rely on?
|
cells and factors contributed from cells other than chondrocytes
|
|
|
What is an example of extrinsic joint repair?
|
Microfracture, Defects in the subchondral bone supply a source of MSC and progenitor cells to the defect
|
|
|
How are defects larger than 5mm in the articular cartilage repaired?
|
extrinsic repair mechanisms.
|
|
|
What hinders Extrinsic repair mechanisms?
|
depleted aggrecan and type 2 collagen and poor reformation of the calcified cartilage layer and subchondral bone
|
|
|
Collagenase MMPs:
|
interstitial collagenase (MMP-1), neutrophil collagenase (MMP-8), collagenase 3 (MMP-13)
|
|
|
What TIMP inhibit MMP-1 (interstitial collagenase)?
|
1>2
|
|
|
Function of MMP-8 (neutrophil collagenase)?
|
Degrade type 2 collagen, aggrecan, link protein
|
|
|
What TIMP inhibit MMP-8 (neutrophil collagenase)?
|
1,2
|
|
|
Function of MMP-13 (collagenase 3):
|
degrade collagen type 2, 4, 9, 10, aggrecan, fibronectin
|
|
|
What TIMP inhibit MMP-13?
|
3
|
|
|
Examples of stromolysin MMPs:
|
MMP-3 (stromolysin-1) , MMP-10 (stromolysin-2)
|
|
|
Function of MMP-3 (stromolysin-1):
|
degrade aggrecan, fibronectin, denature collagen type 2
|
|
|
What TIMP inhibit MMP-3:
|
1>2
|
|
|
Function of MMP-10 (stromolysin-2):
|
degrade collagen type 4, 9, 10, 11, procollagen, link protein, decorin, elastin, laminin
|
|
|
What TIMP inhibits MMP-10?
|
3
|
|
|
Examples of gelatinase MMP:
|
MMP-2, MMP-9
|
|
|
Function of MMP-2:
|
denature collagen type 2, 10, 11, elastin
|
|
|
What TIMP inhibits MMP-2?
|
2>1
|
|
|
Function of MMP-9:
|
degrade aggrecan, fibroconectin, collagen type 9 & 11, proollagen, link protein, decorin, elastin
|
|
|
what is the pathogenic mechanism of perarticular osteophytosis?
|
Enchochondral ossification occurring at bony margin of unknown cause, possible repair attempt
|
|
|
what is the pathogenic mechanism of asymmetric joint space thinning?
|
Cartilage degeneration and loss usually at areas of weight bearing or high stress
|
|
|
what is the pathogenic mechanism of subchondral sclerosis?
|
Deposition of new bone as a response to change in force transmission and from healing of trabecular microfractures, significant sclerosis corresponds to full thickness cartilage loss
|
|
|
what is the pathogenic mechanism of subchondral lysis?
|
Possibly pressure necrosis from synovial fluid gaining access to subchondral plate via fissues or pressure necrosis from trauma
|
|
|
what is the pathogenic mechanism of osteochondral bodies?
|
Disintegration of joint surfaces or fractured osteophytes
|
|
|
what is the pathogenic mechanism of advanced remodeling/ anylosis?
|
Articular response to advance degeneration, environment more consistent with a fracture than a synovial joint
|

