![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
162 Cards in this Set
- Front
- Back
|
Goals of fracture stabilization:
|
reduce pain & anxiety facilitating partial weight bearing; prevention of further compromise; immobilization of adjacent joints.
|
|
|
Types of fracture stabilization:
|
Robert jones bandage, splints, casts
|
|
|
Principles of stabilization:
|
prevent soft tissue damage and regional immobilization
|
|
|
How thick are bandage layers when using a splint?
|
1-2cm thick, thicker layers allow movement of bone fragments or splint can slip
|
|
|
What are the 4 regions of fracture stabilization?
|
1- hoof to distal MC 2- distal MC to distal radius or tarsus 3- distal radius or tarsus to elbow or stifle 4- elbow to distal scapula or proximal to stifle
|
|
|
How are fractures of the phalanges or distal sesamoid bone immobilized?
|
Cast or dorsal splint, for P2 or P1 fractures the limb is immobilized in a straight line including MC/MT3, using either a heel wedge or a commercially available splint
|
|
|
How are fracture of the MC/MT, carpal, or tarsal bone immobilized?
|
Cast or caudal splint from hoof to elbow or stifle
|
|
|
How are fractures of the radius and tarsus immobilized?
|
Cast or lateral splint from foot to point of shoulder or hip
|
|
|
How are fractures of the ulna immobilized?
|
Caudal splint extending carpus from fetlock to elbow
|
|
|
Long bones regions:
|
long, narrow central portion is the diaphysis. The widened ends are the epiphysis. The metaphysis is the transition between the epiphysis and the diaphysis. The physis is the growth plate, located between the epiphysis and the metaphysis
|
|
|
How does longitudinal growth of long bones occur?
|
endochondral ossification
|
|
|
How are chondrocytes arranged for endochondral ossification?
|
longitudinal columns which are parallel to the long axis of the bone
|
|
|
Zones of ossification:
|
From closest to the epiphysis to the diaphysis the zones are the zone of resting cartilage, the zone of proliferation, the pre-hypertrophic zone, the hypertrophic zone, and the zone of calcification
|
|
|
Describe zone of resting cartilage:
|
least metabolically active, and contains active chondrocytes
|
|
|
Describe zone of proliferation:
|
chondrocytes divide in a plane perpendicular to the long axis of the bone to increase bone length
|
|
|
Describe pre-hypertrophic zone:
|
chondrocytes become round and become encased in extracellular matrix
|
|
|
Describe hypertrophic zone:
|
chondrocytes stop dividing, increase in size and hypertrophy
|
|
|
Describe zone of calicification:
|
hypertrophied chondrocytes are replaced by mineralized bone and bone marrow
|
|
|
How does replacement of hypertrophied chondrocytes occur?
|
vascular invasion, resorption of cartilaginous maxtrix, recruitment of osteoblasts, osteoblasts deposit bone matrix
|
|
|
Where is cortical bone thickest/ thinnest?
|
diaphysis and thinnest in the metphysis, epiphysis, and in cuboidal bones
|
|
|
How do cortical and trabecular bone differ?
|
Porosity, trabecular bone is more metabolically active, trabecular bone has more toughness
|
|
|
What are cortical osteon called?
|
haversian systems
|
|
|
What are trabecular osteons called?
|
Packets
|
|
|
Describe Haversian systems:
|
cylindrical and the walls are formed of concentric, plate-like layers of bone called lamellae
|
|
|
How do trabecular lamellae differ from cortical lamellae?
|
semilunar instead of circular
|
|
|
What reflects different biomechanical properties of lamellae?
|
different arrangements of collagen fibrils
|
|
|
What are cement lines?
|
spaces between osteons
|
|
|
When do primary osteons form?
|
during appositional bone growth, which is growth resulting in increased bone thickness
|
|
|
When do secondary osteons form?
|
created throughout life when osteoblasts deposit new bone at the end of bone tunnels
|
|
|
What is the structure of periosteum?
|
The outer fibrous layer is composed of fibroblasts, collagen and elastin fibers, and a nerve-microvascular network. The inner cambium layer contains adult MSC, differentiated osteogenic cells, osteoblasts, fibroblasts, microvessels, and sympathetic nerves
|
|
|
What layer of the periosteum provides cells for fracture healing and appositional bone growth?
|
The inner cambium layer
|
|
|
How is periosteum attached to the bone?
|
with sharpey’s fibers, which are thick collagenous fibers
|
|
|
Describe endosteum:
|
on the inner surface of the cortical and trabecular bone, as well as blood vessel canals called volkman’s canals
|
|
|
Arterial supply to the bone:
|
nutrient arteries, metaphyseal arteries, and periosteal arterioles
|
|
|
Venous drainage from the bone:
|
emissary veins, nutrient veins, cortical channels, and periosteal capillaries
|
|
|
Direction of venous flow in the diaphysis:
|
from the medullary cavity toward the cortex
|
|
|
Direction of arterial flow in the diaphysis:
|
70% is from the medullary cavity toward the cortex and 30% is from the cortex toward the medulla
|
|
|
What is the blood supply to the medulla and cortical bone:
|
Endosteal circulation supplies the medullary cavity and inner 2/3 of cortical bone and periosteal circulation supplies the outer 1/3 of the cortical bone
|
|
|
Composition of bone:
|
40% inorganic, 30% organic, and 25% water
|
|
|
What is the inorganic portion of bone?
|
predominately hydroxyapatite
|
|
|
What is the organic portion of bone?
|
predominately type 1 collagen
|
|
|
What is the inorganic portion of bone responsible for?
|
stiffness and strength of bone
|
|
|
What is the organic portion of bone responsible for?
|
ductility and toughness of bones
|
|
|
What is ductility?
|
ability to plastically deform without fracture
|
|
|
What is toughness?
|
ability to absorb energy
|
|
|
Arrangement of type 1 collagen:
|
triple helix composed of 2 alpha 1 chain and 1 alpha 2 chain. The helixes form linear molecular that align parallel to each other to form fibrils. Grouped collagen fibrils form collagen fibers
|
|
|
Bone cells:
|
osteoblasts, osteocytes, and osteoclasts
|
|
|
Where are osteoblasts derived from?
|
MSC
|
|
|
Function of osteoblasts:
|
synthesize collagenous organic matrix, regulate mineralization, and release enzymes to destroy mineralization inhibitors
|
|
|
What happens to osteoblasts after bone formation?
|
50-70% of osteoblasts undergo apoptosis, the remainder become osteocytes or bone lining cells
|
|
|
Where are osteocytes derived from?
|
osteoblasts that have become embedded in matrix within canaliculi of mineralized bone
|
|
|
Function of bone lining cells?
|
regulate mineral ion influx and efflux, transmit stress signals from bending and stretching of bone and can redifferenciate back to osteoblasts
|
|
|
Where are osteoclasts derived from?
|
precursor cells of the monocyte-macrophage lineage
|
|
|
What regulates osteoclast development?
|
numerous cytokines and hormones, but the most essential are receptor activator of nuclear factor KB (RANKL) and macrophage colony stimulating factor (M-CSF)
|
|
|
Function of activated osteoclasts:
|
secrete hydrogen ions to lower pH and mobilize bone mineral, secrete enzymes to digest organic matrix
|
|
|
What is the result of osteoclastic activity?
|
formation of saucer-shaped Howship lacunae in cortical bone
|
|
|
Load:
|
externally applied forces
|
|
|
What occurs when load is applied to bone?
|
alters the shape and size of the bone by deformation
|
|
|
What are structural properties?
|
mechanical properties that depend on the dimension of the bone
|
|
|
What reports the relationship between load and displacement when testing structural properties?
|
load deformation curve
|
|
|
What are the portions of the load deformation curve?
|
toe region, yield point, elastic region, plastic region, failure point
|
|
|
What is the toe region of a load deformation curve?
|
initial curved segment that is the high deformation region of the usual response of bone to physiologic forces
|
|
|
What is the elastic region of the load deformation curve?
|
characterizes the bone’s ability to resist deformation, and the bone retains is ability to return to its original form when the load is removed
|
|
|
What is the slope of the curve at the elastic region referred to?
|
stiffness of the bone
|
|
|
What is the yield point of the load deformation curve?
|
segues from elastic region to the plastic region
|
|
|
What is the plastic region of the load deformation curve?
|
where the bone does not return to its original form and is permanently deformed
|
|
|
What is the ultimate load?
|
load beyond which the bone loses all capacity to withstand increased force
|
|
|
What are material properties?
|
mechanical properties of the composite materials
|
|
|
What is stress?
|
intensity of force divided over the area that the stress acts on (force over an area)
|
|
|
Types of stresses:
|
normal or shear
|
|
|
When do normal stresses occur?
|
when forces are applied perpendicular to the surface of the bone
|
|
|
When do shear stresses occur?
|
when forces are applied parallel to the cross section of the bone
|
|
|
Examples of normal stresses:
|
Tension is a positive normal stress, compression is a negative normal stress
|
|
|
What is strain?
|
change in dimension of the bone divided by the original dimension of the bone (change in length over the initial length)
|
|
|
Types of strain:
|
normal or shear
|
|
|
When does normal strains occur?
|
when forces are applied perpendicular to the cross-section of the bone
|
|
|
When does shear strains occur?
|
when forces are applied parallel to the surface of the bone
|
|
|
What is Poisson’s ratio?
|
ratio of lateral normal strain or to longitudinal normal strain
|
|
|
What load is bone strongest & weakest in?
|
strongest in compression, weaker in shear, and weakest in tension
|
|
|
What is tension?
|
load applied equal and opposite to both ends of the bone
|
|
|
When does maximum stress occurs with tension?
|
perpendicular to the tension load
|
|
|
Examples of tension fractures:
|
transverse, such as olecranon fractures, PSB fractures, patellar fractures, and calcaneal fractures
|
|
|
What is compression?
|
load applied equal and toward each other (force is aligned concentric or in line with the body column)
|
|
|
When does maximum stress occur with compression?
|
perpendicular to the compression load
|
|
|
Orientation of compression fracture:
|
fracture offset 45 degrees to the maximum shear force. Compression fractures are oblique
|
|
|
What is torsion?
|
rotational displacement of the ends around a central axis
|
|
|
Where does failure occur in torsion?
|
failure begins parallel to the neutral axis of the bone but continues 45 degrees offset from the neutral axis
|
|
|
Configuration of rotational fractures?
|
Spiral
|
|
|
What is bending?
|
combination of tension and compression. Tension is on 1 side and bending is on the opposite site. force is applied eccentric (off center) with the body column creating a compressive force on 1 side and a tension force on the other side
|
|
|
Examples of bending fractures:
|
fracture that occurs at the top of a cast or when a horse steps in a hole
|
|
|
Where does bending failure occur?
|
begins on the tension surface toward the compression surface, when the shear force is greater than the tension force, the fracture propagates 45 degrees to the neutral axis
|
|
|
Configuration of bending fractures:
|
transverse with a butterfly fragment on the compression side
|
|
|
What is shear?
|
load applied parallel to 1 surface of the bone, deforming the bone angularly
|
|
|
Example of shear fracture:
|
Physeal fractures
|
|
|
What is the relationship of rate of load application and energy?
|
Bones that are loaded rapidly fail at higher loads and release more energy
|
|
|
How does direct or primary fracture healing occur?
|
osteonal reconstruction, osteoblastic bone production begins without an intermediary tissue because there is anatomic reduction, rigid internal fixation, no inter fragmentary strain, and good vascular supply
|
|
|
Role of Haversian systems in fracture repair?
|
fragments regenerate by growth of secondary osteons and intramembranous bone formation
|
|
|
How does indirect or secondary fracture healing occur?
|
endochondral bone formation, occurs when the fragments are not sufficiently immobilized or approximated
|
|
|
Role of inflammatory phase:
|
destruction of matrix and necrotic debris, hematoma formation, soft callus formation and angiogenesis
|
|
|
Composition of soft callus:
|
type 3 collagen and stromal cells that differentiate into chondrogenic and osteogenic cells, creating a template for a calcified callus
|
|
|
Initial blood supply to the callus:
|
from tissues that surround the callus, not periosteal vessels
|
|
|
Role of repair phase:
|
mineralization of the soft callus to form woven bone by endochondral ossification and intramembranous ossification, mineralized callus forms a bridging callus, which is clinical union
|
|
|
What determines the size of the callus?
|
proportional to the amount of motion at the fracture site
|
|
|
Role of remodeling phase:
|
continued replacement of mineralized cartilage to woven bone, and remodeling of woven bone to lamellar bone.
|
|
|
Final product of the remodeling phase:
|
regenerated bone with organic and mineral components aligned to resist physiologic stress and strain
|
|
|
Radiographic evidence of fracture repair:
|
Within the first week of fracture, fracture margins appear sharp on radiographs. Once the inflammatory phase has been established, within 2 to 3 weeks of fracture, the fracture gap widens radiographically. During the repair and remodeling phases, calluses are evident radiographically. Bony union is radiographically characterized by obliteration of the fracture line and cortical bridging of the fracture gap
|
|
|
Complications of fracture healing:
|
infection, fixation failure, delayed or failure of bone union, laminitis, and angular limb deformities in foals
|
|
|
Healing time for fractures:
|
Adult fractures should heal within 4 months and foal fractures should heal within 3 months
|
|
|
Delayed union:
|
healing is progressing but at a slower rate than normal
|
|
|
Non-union:
|
repair stops before bone structure is restored
|
|
|
Why do delayed or non-union occur?
|
infection, poor reduction or immobilization, and soft tissue injury
|
|
|
Types of non-union:
|
hypervascular, oligotrophic, avascular, comminuted, and defect
|
|
|
How is non-union treated?
|
debridement, grafting, and improving stability
|
|
|
When do implant complications occur?
|
when the construct experiences forces that exceed the implants strength or when healing takes longer than the implant’s fatigue life. Infection or bone failure can result in loosening of implants
|
|
|
Distraction osteogenesis:
|
formation of new bone by controlled gradual traction on each side of the bone ends to stimulate regeneration and progressive growth of bone and soft tissues
|
|
|
What does distraction osteogenesis require?
|
fixation stability, periosteal and medullary vascular supply, minimal soft tissue injury and physiologic use of the bone
|
|
|
Phases of distraction osteogenesis:
|
latency period is between the time of bone transection and initiation of distraction, the activation period is the period of bone growth during application of traction, and the consolidation period is the period that the distraction devise remains in place to provide rigid fixation as the new bone matures
|
|
|
What can stimulate bone healing?
|
application of autogenous or allogenic bone grafts, pulsed ultrasonic or electromagnetic fields, use of biodegradable bone cements during fixation, administration of bone morphogenic proteins, growth factors or cytokines, or administration of MSC
|
|
|
Types of autogenous or allogenic grafts:
|
corticocancellous bone, demineralized bone matrix, or cortical bone
|
|
|
Benefits of bone grafts:
|
allow osteogenesis by provision of cells, osteoinduction by inducing bone formation by host cells, and osteoconduction by providing a scaffold for bone formation
|
|
|
Types of metallic instrument materials:
|
stainless steel, aluminum, aluminum alloy, titanium alloy
|
|
|
Use of martensitic stainless steel (instruments):
|
cutting instruments (drills, taps, countersinks, reamers, chisels, bone-cutting forceps), non-cutting instruments (screwdrivers, wrenches)
|
|
|
Use of precipitation hardenable stainless steel (instruments):
|
non-cutting instruments that require moderate hardness
|
|
|
Use of austenitic stainless steel (instruments):
|
non-cutting instruments (drill guides, clamps, hollow sleeves, springs, washers)
|
|
|
Characteristics of aluminum instruments:
|
low strength, highly ductile (malleable), non-magnestic, light-weight
|
|
|
Difference between aluminum and aluminum alloy instuments:
|
alloys have more strength and less ductility (malleability)
|
|
|
Use of aluminum alloy (instruments):
|
depth gauges, IM nail insertion instruments, hollow external fixation rings, graphic case modules, screw racks
|
|
|
Benefits of anodizing:
|
corrosion resistance, increase surface hardness
|
|
|
Galling:
|
adhesive wear that occurs when 2 metals rub together
|
|
|
Use of titanium alloy (instruments):
|
non-cutting (external fixation components, small diameter guide wires, aiming instruments)
|
|
|
Types of metallic implant materials:
|
stainless steel, titanium, titanium alloy, cobalt based alloys
|
|
|
Composition of 316L implant quality stainless steel:
|
18% chromium, 14% nickel, 2.5% molybdenum
|
|
|
Why are implants components of different materials not used together?
|
Galvanic corrosion, an accelerated form of corrosion can occur
|
|
|
What is cold working?
|
Metal working to permanently deform material at room temperature to increase strength
|
|
|
What provides corrosion resistance to stainless steel implants?
|
Chromium oxide film, called passive layer
|
|
|
What is electropolishing?
|
Surface treatment to stainless steel that decreases surface roughness and improves corrosion resistance
|
|
|
When is unalloyed titanium used for implants?
|
When metal sensitivity is thought to be a risk
|
|
|
Benefit of unalloyed titanium implants:
|
soft tissue integration at the surface results in less bacterial colonization, improved vascularity of tissue adjacent to implant, decreased capsule formation
|
|
|
Disadvantage of unalloyed titanium implants:
|
hard to remove because of integration into tissues
|
|
|
Advantages of unalloyed titanium implants compared with stainless steel:
|
less MR artifact, lower density, lower modulus of elasticity
|
|
|
What is modulus of elasticity?
|
Stress per unit strain in the elastic region
|
|
|
How does a high modulus of elasticity of an implant affect the bone?
|
transfers less strain to the bone, which may not be beneficial because bone requires some stress for healing
|
|
|
How do titanium alloys compare with titanium?
|
Higher tensile strength, lower ductility, similar modulus of elasticity, similar density
|
|
|
What are cobalt base alloys used for?
|
Prosthetic implants
|
|
|
How do cobalt based alloys compare with stainless steel?
|
Higher modulus of elasticity
|
|
|
What is implant fatigue?
|
Fracture under repeated or fluctuating stresses having a maximum valve less than the ultimate tensile strength of the material
|
|
|
What is fatigue cycle?
|
Time interval during which stress is regularly repeated
|
|
|
What is endurance limit?
|
Maximum stress below which a material can endure an infinate number of stress cycles
|
|
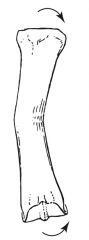
|
bending
|
|
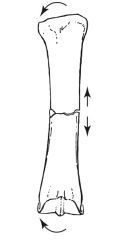
|
bending, fails first in tension (transverse) then compression to form a butterfly fragment
|
|
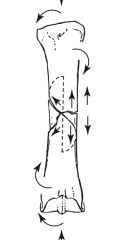
|
compression and rotation result in communution
|
|

|
compression
|
|
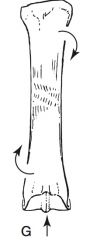
|
compression & torsion
|
|
|
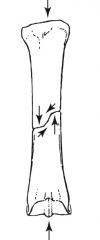
compression (oblique fracture)
|
|
|
shear
|
|

|
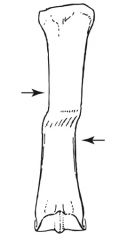
spiral fracture from torsion
|
|
|
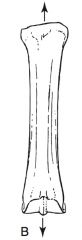
tension
|
|
|
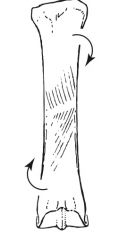
torsion
|
|
|
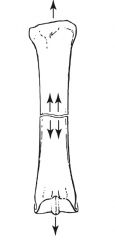
tension fracture transeverse
|
|
|
define viscoelastic
|
energy absorbed by the bone relies on the rate in which the bone was loaded. with increase rate of loading (application of a load quickly) the bone has more stiffness (slope of curve is steeper) with a slow rate of loading (application of a load slowly) the bone is less stiff high rate of loading, more stiffness, means more energy release at failure point
|
|
|
define anisotropy
|
strength and stiffness of bone depend on the direction the bone is loaded. the bone is more stiff and strong when loaded parallel to its osteonal system and the bone is less stiff and strong when the load is applied perpendicular to the osteonal system
|
|
|
what bone only experiences compression
|
MC/MT3
|

