![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is an element |
A substance that can't be broken down into any simpler substances. |
Can't be broken down.... |
|
|
What is a compound |
Two or more different atoms chemically joined together. |
|
|
|
What is a mixture |
A mixture of atoms. It is not a pure substance |
|
|
|
What are sub atomic particles |
Particles inside an atom |
|
|
|
Name sub atomic particles |
Protons, neutrons, electrons |
|
|
|
Charge of protons |
Positive charge |
|
|
|
Charge of electrons |
Negative charge |
|
|
|
Charge of neutrons |
Neutral charge |
|
|
|
Location of proton |
Inside nucleus |
|
|
|
Location of electron |
Outside nucleus |
|
|
|
Location of neutron |
Inside nucleus |
|
|
|
What is the atomic number |
The number of protons or electrons in an element. |
|
|
|
What is the Mass number |
Atomic number plus Neutron number. |
|
|
|
Nuclide Notation |

|
|
|
|
What is an isotope |
Isotopes are elements with the same atomic number but different mass number. |
|
|
|
Valency pictures |
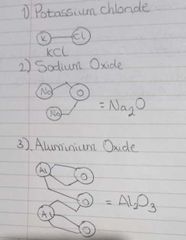
Examples |
|
|
|
Cross over method (finding the formula) |

SVSDF |
|
|
|
Page 8 of data booklet exceptions |
Ammonium & Hydroxide |
|
|
|
Transition metal valencies |
If the valency is not told it is taken down as 2. |
|
|
|
Example of transitional valency |

|
|
|
|
What is the valancy |
The valency is the combining power of the element |
|
|
|
RAM examples |
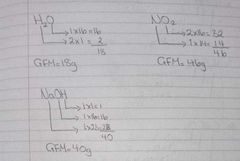
|
|
|
|
Harder RAM questions |
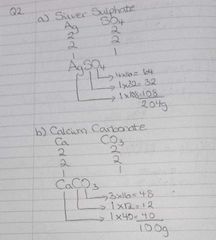
Finding the formula then finding the gram formula mass. |
|
|
|
Formula writing instructions |
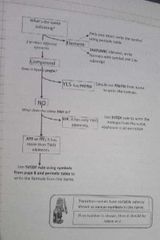
|
|
|
|
Formula writing example |

|
|
|
|
What is a balanced equation |
A balanced equation is when Tue number of atoms for each element is the same on the left and right hand side of the equation. |
|
|
|
Example of balanced equation |
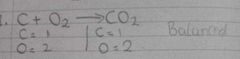
|
|
|
|
Example of 2 unbalanced equations |
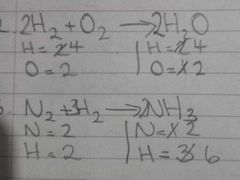
|
|
|
|
What is ionisation |
The addition or subtraction of an electron to create an ion. |
|

