![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Matter
|
Anything that has both mass and volume.
|
|
|
|
Atomic Number
|
the number of protons or electrons in an atom
|
|
|
|
Atomic Mass or Mass Number
|
the number of protons + the number of neutrons in an atom
|
the number of particles found in the nucleus of an atom
|
|
|
Nucleus
|
the center of an atom containing protons and neutrons
|
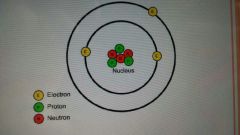
|
|
|
protons
|
positively charged particle
|
|
|
|
Neutrons
|
particles with NO charge
|
circling the nucleus
|
|
|
electrons
|
very very tiny negatively charged particles
|
|
|
|
metals
|
elements that are good conductors of heat and electricity
|
|
|
|
nonmetals
|
elements that are poor conductors of heat and electricity
|
|
|
|
metalloids
|
elements with some characteristics of metals and some of nonmetals
|
|
|
|
Atoms
|
The arrangement of the atoms give matter it's characteristics and properties
|
physical and chemical properties are used to identify a substance and predict how it will behave
|
|
|
Physical Properties
|
Characteristics that can be observed or measured with out changing the identity of the material
|
density length color shape mass volume melting point boiling point
|
|
|
Chemical Properties
|
any property that produces a change in composition of matter
|
A substance ability to combine or react with other substances
change in color gas production rust or tarnish |
|
|
element
|
a substance that cannot be broken down into simpler substances. something made of onlt one kind of atom
|
the smallest part of an element that still has the properties of that element
|
|
|
Compound
|
A substance made up of atoms of 2 or more elements that are chemically combined. They alwayscombine in the same ratio. Compunds have properties that are different from the properties of their single atoms
|
elements and conpounds are pure substances
|
|
|
Mixture
|
a combination of two or more pure substances that are not chemically combined
|
|
|
|
Heterogeneous Mixtures
|
are not distributed or spread out evenly. You can see the different parts of the mixtures
|
candy assortment
|
|
|
Homogeneous mixture
|
are evenly distributed and you cannot see the different parts of the mixture
|
gatorade
|
|
|
Solid
|
define shape and volume
|
state of matter
|
|
|
liquid
|
change shape but not volume.
|
state of matter
|
|
|
gas
|
change both shapr and volume
|
state of matter
|

