![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
What is an isotope?
|
An atom of the same element but with a different number of neutrons.
|
|
|
What is Relative atomic mass?
|
The Average mass of an atom compared to the mass of a carbon-12 atom( compared to 1/12 of a carbon-12 atom)
|
|
|
What are the five staged is mass spectrometry?
|
Vapourisation
Ionisation Acceleration Deflection Detection |
|
|
Vapourisation
|
The sample is heated to turn it into a gas.
|
|
|
Ionisation
|
The gas is passed under an electron gun where an electron will be knocked off the atom leaving it with a 1+ charge.
|
|
|
Acceleration
|
-vely charged plates are used to direct the +ve ions and increase there speed.
|
|
|
Deflection
|
An electromagnet created a magnetic field to deflect the ions.
Heavy= small deflection Light= large deflection |
|
|
Detection
|
The magnetic field is increased to ions hit the detector in order of increasing mass.
The ions hit the detector, gain and electron and induce a current proportional to the abundance of that ion. |
|
|
What is mass spectrometry used for?
|
To identify the different isotopes that make up an element. Different isotopes are detected separately because they have different masses.
|
|
|
What is the order of the sub levels in electron shells?
|
S
P D F |
|
|
S can hold...?
|
2 electrons.
|
|
|
P can hold...?
|
2 Electrons each but always come in groups of three in an energy level therefore...
6 in total. |
|
|
D can hold...?
|
2 electrons each but always come in groups of five in an energy level so therefore...
10 in total. |
|
|
Which is easiest to fill 3D or 4S?
|
4S
|
|
|
Three rules for allocating electrons to atomic orbitals?
|
- Atomic orbitals with lower energy are filled first
- Atomic orbitals of the same energy fill singly before pairing starts - No atomic orbital can hold more that 2 electrons |
|
|
Ionisation
|
The process by which atoms lose or gain electrons.
|
|
|
The first ionisation energy is...
|
the energy needed to remove one electron from each atom in one mole of gaseous atoms to produce one mole of gaseous 1+ions.
|
|
|
What are the three factors affection ionisation energy?
|
Nuclear charge
Distance from nucleus Shielding |
|
|
Explain Nuclear charge in terms of affecting ionisation energy?
|
The more protons in the nucleus the stronger the attraction for the electrons therefore the harder to lose an electron.
|
|
|
Explain distance from the nucleus in terms of affecting ionisation energy?
|
Attraction falls with distance. The further away an atom from the nucleus the easier it is to lose.
|
|
|
Explain shielding in terms of affecting ionisation energy?
|
As the number of electrons between the outer electrons and the nucleus increases the attraction between the outer electrons and the nucleus decreases.
|
|
|
A high ionisation energy means...?
|
there is a high attraction between the nucleus and the electron.
|
|
|
Down a group the ionisation energy...?
|
decreases. (ie electrons are easier to remove)
This is because the atoms get bigger meaning that there is a farther distance between the electrons and the nucleus. also there are more electrons down a group so outer electrons are more shielded. |
|
|
Across a period ionisation energy...?
|
increases. (ie electrons are harder to remove)
This is because the number of protons is increasing therefore there is stronger nuclear attraction between the nucleus and the electrons. |
|
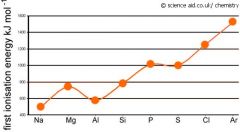
Explain the drops between groups 2 and 3, and groups 5 and 6?
|
Groups 2 and 3
Aluminium's outer electron shell is a 3P orbital which means it is further from the nucleus and there is also extra shielding meaning it is easier to lose. Groups 5 and 6 Sulphur has 2 electrons in its outer orbital whereas phosphors has one. It is easier for sulphur to lose its electron because of the repulsion between the two. |

