![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
62 Cards in this Set
- Front
- Back
|
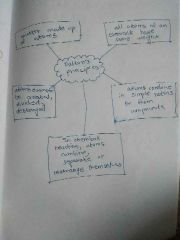
Dalton's principles |
|
|
|
Niel Bohr |
Planetary model (energy of electrons associated with orbit) Hydrogen line spectrum |
|
|
Electron discovery |
J.J Thompson through cathode ray experiment Also discovered electron charge\mass |
|
|
Mass of an electron |
Millikan's oil drop experiment 9.11×10^-28 gram |
|
|
Rutherford |
Gold foil experiment Electrons revolve around neutron |
|
|
Discovery of neutron |
James Chadwick 5 1.675×10^-24 |
|
|
Bohr model |
Electrons in specific energy shells Maximum no. of electrons in each shell-2n^2 Electrons need to gain energy to move away from neutron Outermost energy level contains maximum 8 electrons |
|
|
Atomic number |
Number of protons Identity of element |
|
|
Atomic mass number |
Number of protons + number of neutrons |
|
|
Isotopes |
Same atomic number different atomic mass Same number of protons |
|
|
Average atomic mass |
Weighted average of atomic masses of isotopes Sum of Atomic mass × abundance(decimal form) |
|
|
Valence electrons |
Electrons in outermost shell |
|
|
Change in energy |
∆E= E(final)-E(initial) |
|
|
Atomic spectra |
Ground to excited- energy absorption Excited to ground- energy emission In the form of photons |
|
|
Line of spectra |
n=1, Lyman series,ultraviolet n=2 ,Balmer series , visible n=3 ,Paschen series,infrared |
|
|
Visible light spectra |

|
|
|
Mass spectroscopy |
Separates isotopes of SAME element based on mass |
|
|
Uncertainty principle |
Heisenberg stated- Impossible to know both location AND velocity of an subatomic particle |
|
|
Orbital |
Obtained by wave mechanical model Not related to Bohr's orbit 3D region around nucleus giving probable location of electron |
|
|
Pauli exclusion principle |
No two electrons can have the same set of quantum numbers. Each orbital can hold only 2 electrons |
|
|
Principle quantum number |
Distance of orbital from nucleus As called energy level n=1,2,3 |
|
|
Angular quantum number |
Shape of orbital l=0...(n-1) 0- spherical shaped s orbital 1- dumbell shaped p orbital 2-five orbital orientation d orbital |
|
|
Magnetic quantum number |
Spatial orientation of orbital m= +l...-l |
|
|
Spin quantum number |
Spin in either direction m= +1/2 or -1/2 Each orbital consists of only 2 electrons in opposite spins |
|
|
Hund's rule of maximum multiplicity |
After every orbital is occupied by an electron, pairing will take place |
|
|
Aufbau principle |
An electron occupies the lowest energy orbital that can receive it |
|
|
Filing of orbitals |

|
|
|
Lewis Dot structure |
Dots represent valence electrons |
|
|
Transition elements |
Elements involved with the filling of a d sublevel Starting from Calcium and gallium |
|
|
Transition elements properties |
Form coloured compounds Form complex ions Variety of oxidation states Good catalysts At room temperature- Solids and silvery blue Paramagnetic At least one compound has incomplete d orbital |
|
|
Periodic table discovery |
Mendeleev |
|
|
Periods |
Horizontal rows 1-7 |
|
|
Groups |
Vertical columns 1-18 Elements in a group have same number of electrons in outer shell |
|
|
Stability of compounds |
Half filled or fully filled are most stable Eg: Cr,Cu |
|
|
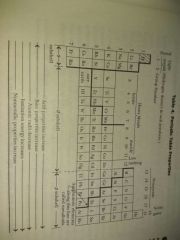
Periodic table properties |
|
|
|
Periodic law |
Properties of elements are a function of the atomic number |
|
|
Most active metal |
Francium |
|
|
Most active non metals |
Fluorine |
|
|
Metalloids |
Elements containing characteristics of metals and non metals Eg: Si, B, As, Te |
|
|
Covalent radius |
Distance between two nuclei/2 |
|
|
Atomic radius |
Decrease from left to right(periods) ( Electrons added to same shell) Increase from top to bottom(groups) ( Shells increase in number) |
|
|
Ionic and atomic radius |
In metals Ionic < Atomic In non metals Ionic > Atomic |
|
|
Electronegativity |
Measurement of strength of atoms with which they attract valence electrons in a chemical bond A value less than two metal Decreases down the group Increases across a period |
|
|
Most electronegative element |
Fluorine |
|
|
Most electronegative element |
Fluorine |
|
|
Most electropositive element |
Francium |
|
|
First Ionisation energy |
Energy required to remove one outer electron |
|

|
The peaks starting from He as they are most stable elements Moving from Li to Ne energy increases Li - only one electron in s orbital can be easily removed Be - two electrons in s orbital is more stable B- a lone electron occupies 2p which can be easily removed Other elements follow the same pattern |
|
|
Beta particle |
Neutron decays into proton and electron High velocity, low energy Range : 12 cm Shielding needed : 1cm aluminium Weak interactions 100 Ionisation Neutron converted to proton |
|
|
Alpha particle |
Reduction Atomic number by 2 Atomic weight by 4 amu Positively charged High energy, relative velocity 1,00,000 ionisation Requires paper like shielding Range: 5 cm |
|
|
Gamma radiation |
Emitted together after beta radiation Same velocity as visible light Shielding of 13 cm lead Weak interactions |
|
|
Methods of detection of radioactive emission |
Photographic plate - beta and gamma Scintillation counter- alpha particle Geiger counter |
|
|
Half life |
Time required for half of the atoms of a radioactive nuclide to decay to radon |
|
|
Transmutation |
Conversion of an element to a new element Radium loses an alpha particle , which gains two electrons and becomes neutral helium |
|
|
Changes occurring during radioactive reactions |

|
|
|
Nuclear fusion |
Combination of very light nuclei to make a heavier nucleus |
|
|
Nuclear fission reaction |
Division of a heavy nucleus into lighter nuclei |
|
|
Energy shell |
A collection of orbitals of similar size |
|
|
Positron emission |
Proton converted to neutron Atomic number decreases by 1 Positron( positively charged particle except proton , has the same mass as that of electron) emitted |
|
|
De Broglie |
Postulated that matter can act as both particle and wave |
|
|
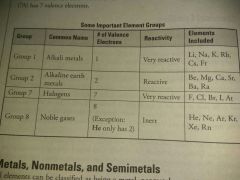
Element groups |
|
|
|
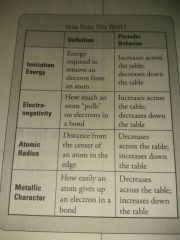
Periodic trends |
|

