![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
4 Cards in this Set
- Front
- Back
|
Structure Of The Atom
|
Atoms Contain Three Sub-atomic particles called protons, neutrons and electrons. The protons and neutrons are found in the nucleus at the centre of the atom. the nucleus is very small than the atom as a whole. the electrons are arranged in shells around the Nucleus. |
|
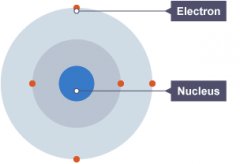
Atom Structure
|

The Periodic Table
|
|
|
Properties of Sub-atomic Particle
|

The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral overall. |
|
|
Properties of Sub-atomic Particles Continued
|
Atoms can lose or gain electrons. when they do form charged particles named ions - If an Atom loses one or more electrons, it becomes positively charged ion - If an Atom gains one or more electrons it becomes a negatively charged Ion |

