![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
224 Cards in this Set
- Front
- Back
|
What is the shape of a molecule determined by? |
it is determined by the number of pairs of electrons in the outer shell of the central atom. |
|
|
Which of the following causes more repulsion:
-LONE PAIRS -BONDING PAIRS |
LONE PAIRS |
|
|
Define VESPR (valence electron shell pair repulsion): |
Valence Shell Electron Pair Repulsion Theory (VSEPR) is a molecular model to predict the geometry of the atoms making up a molecule where the electrostatic forces between a molecule's valence electrons are minimized around a central atom. |
|
|
What is the name of the shape that a molecule forms when it forms 4 single bonds and what is its angle? |
Tetrahedral, 109.5 degrees |
|
|
What is the name of the shape of a molecule that forms 2 single bonds with no lone pairs? What would you call it if it had lone pairs? Give their angles: |
Linear (no lone pairs) 180 degrees
Bent (with lone pairs 120 degrees/104.5 in h20 |
|
|
What is the name given to molecules that form 3 single bonds with no lone pairs and lone pairs? What are their angles? |
Trigonal Plannar (no lone pairs) 120 degrees Trigonal Pyramidal (lone pairs) 107 degrees |
|
|
What is the name given to molecules that form 5 single bonds ? What are their angles? |
Trigonal bipyramidal 120 degrees and 90 degrees |
|
|
What is the name given to molecules that form 6 single bonds? What are their angles? |
Octahedral all bonds 90 degrees |
|
|
Define Allotropes |
Allotropes are different forms of the same element in the same state. |
|
|
What are the allotropes of carbon? |
-Graphite -Diamond -Fullerenes |
|
|
Describe how Diamond is structured, its properties and why it is so strong.
|
Because it forms very strong covalent bonds in a tetrahedral crystal lattice structure, it has: 1) a very high melting point over 3800K 2)very hard (used for drill bits) 3)vibrations travel easily through its lattice so it is a good thermal conductor 4) can't conduct electricity (localized electrons) 5) won't dissolve solvents |
|
|
Describe how Graphite is structured, its properties and why it is so strong. |
1) is a macromolecular structure arranged in sheets of flat hexagons covalently bonded to 3 carbon atoms each + 1 delocalised electron. 2) weak bonds between layers making it slippery and easily broken. 3)conducts electricity (delocalised electrons) 4)very high melting point (3900K) due to strong covalent bonding. 5)insoluble in any solvent |
|
|
Describe how Fullerenes are structured and give an example of a fullerene: |
1)are made of carbon and can be structured to form hollow balls or tubes. 2) are nanoparticles 3) are soluble in organic solvents 4)since they are hollow they can cage other molecules that can deliver drugs. 5) nanotubes are very small can be used for tennis rackets and circuits (they conduct electricity). They can also be capped at the ends. Example of a fullerene: Buckministerfullerene C60 (found in soot). |
|
|
Debate whether or not you think Nanotechnology is safe? Why or why not? |
nanotechnology can be quite risky. For example: Sunscreens. Many of them contain nano particles such as zinc oxide or titanium dioxide. These reflect UV radiation off the skin (which is useful as is reduces skin cancer) however sometimes they pass through the skin and into cells to damage them. However, scientists are still not sure. |
|
|
What is the problem with using models of bonding (dot and cross diagrams)? |
They only illustrate how the atoms in a compound share their electrons. It can't explain bond lengths or the overall shape of the molecule. Very few molecule are purely ionic or covalent. |
|
|
What type of bond is purely covalent? |
Diatomic bonds between like atoms. |
|
|
Define Electronegativity: |
the ability or tendency to attract bonding electrons in a covalent bond. |
|
|
What is the most electronegative element? |
Flourine |
|
|
Which has the shortest bond length and has the highest bond enthalpy? C-C C=C or c≡c |
c≡c since the electron density is greater, the bond length is shorter and the bond enthalpy is greater. |
|
|
Define bond length: |
This is the distance between two or more nuclei in a molecule. |
|
|
Name the 3 types of Intermolecular forces:
|
-Instantaneous Dipole-induced Dipole (London forces) - Permanent Dipole-dipole -Hydrogen bonding |
|
|
How do london forces keep atoms and molecules attracted to each other? |
Electrons in charge clouds are always moving. This means that at any moment the electrons can become distributed to one side than the other making it a temporary dipole which can induce other dipoles in molecules and atoms which hence, attract each other. |
|
|
What affects the strength of London forces in terms of Boiling/melting points? |
-larger molecules (they have larger electron clouds which means they have a bigger exposed electron cloud which can bond to other atoms) -shape of the molecule (chained has stronger london forces vs. branched). |
|
|
What are permanent Dipole-dipole forces? |

This occurs exclusively with molecules that are permanently polar not with temporary dipoles that have been caused by a shift in electrons. An attraction here forms between the slightly negative/positive ends of molecules which attach one another |
|
|
How could you test if a liquid was polar? |
Place an electrostatically charged rod next to the liquid as it drips in a continuous flow and observe if it suddenly gets closer to the rod (an example of a polar liquid to experiment with is water). |
|
|
What is Hydrogen bonding and what is it limited to bonding with? |
this only occurs when Hydrogen bonds with Nitrogen, Oxygen or Flourine (NOF) as they are very electronegative and draw electrons away from the Hydrogen. They are also the strongest Intermolecular force giving most liquids with this sort of bonding extremely high boiling temperatures. |
|

Explain the trend in the boiling points of the hydrogen halides in this table. |
The HF has the highest since its bonded using Hydrogen bonding. The rest of the molecules have their anion increasing in size which results in stronger london forces giving it a higher boiling point as you go down the group. |
|
|
Why does ice float in water? |
Ice has more hydrogen bonds than liquid water. The hydrogen bonds are also quite long so the H2O molecules are further apart making it less dense than water. |
|
|
What are the two main types of solvent and give an example of each: |
-POLAR (e.g water) -NON-POLAR (e.g hexane) |
|
|
What must happen in order for a solute to effectively dissolve in a solvent? |
the new bonds formed must be the same as or greater than the strength of the bonds that are broken. |
|
|
What is hydration and how does it happen? |
This is the process in which ionic substances (such as NaCl) dissolve in polar solvents such as water. This occurs when the the ions are attracted to the oppositely charged ends of the water molecules which are pulled away from the rest of the molecule breaking it apart. |
|
|
Give an example of an ionic substance that will NOT dissolve in a polar solvent like water and explain why. |
Al2O3 is insoluble because the bonds between the ions are stronger than the bonds it will form with water. |
|
|
Besides ionic substances , what else has the ability to dissolve in polar solvents such as water and how does it occur? |
Alcohols are covalent however they dissolve in water because the polar O-H bonds in an alcohol are attracted to the OH of the water and hydrogen bonds form between the Oxygen and Hydrogen pulling it away from the rest of the molecule. |
|
|
What affects the solubility of an alcohol? |
the carbon chain of the alcohol is insoluble, therefore the longer the carbon chain is, the more insoluble it becomes. |
|
|
What type of compound (besides non-polar compounds) is not soluble in polar solvents such as water? |
2) Halogenoalkanes contain polar bonds but their dipoles are not strong enough to form hydrogen bonds with water. 2) the bonds water forms with itself is stronger than the bonds it would form with the solute therefore it does not dissolve. |
|
|
What do non-polar and polar substances dissolve better in? |
the rule: like dissolves like - substances usually dissolve best in solvents that have similar bonds.
1)Polar solutes - polar solvents 2) Non-polar solutes - non-polar solvents. |
|
|
What is REDUCTION? |
is a gain in electrons |
|
|
What is OXIDATION? |
is a loss in electrons |
|
|
DEFINE REDOX |
this is when both reduction and oxidation occur simultaneously. |
|
|
What is the oxidation of an element |
0 |
|
|
What is the oxidation number of diatomic molecules? |
0 |
|
|
What is the oxidation number of Oxygen and when does it have an exception? |
usually, its -2 but in peroxides its -1 and in Flourides it is +2. |
|
|
What is the oxidation number of Hydrogen and when does it have an exception? |
usually, its +1 but in metal hydrides (e.g NaH) it is -1 |
|
|
What happens to the ionisation energy as you go DOWN group 2? |
it decreases because the 1)number of shells increase. 2)shielding increases 3)distance from nucleus increases which results in less attraction between nucleus and outermost electrons making it easier to remove. |
|
|
How do GROUP 2 elements react with water? |
they react with water to form a metal hydroxide and hydrogen,
M(S)+2H20(l)---------> M(OH)2 (aq) + H2 (g) |
|
|
What happens to the reactivity of group 2 elements as you go down the group? |
it increases in reactivity because the ionisation energies are decreasing. (group 2 metals WANT to loose electrons so it being bigger makes it easier to loose the electrons due to the lack of attraction between the nucleus and outermost shell!) |
|
|
How do GROUP 2 metals react with Oxygen? |
they burn with different flame colors |
|
|
How do GROUP 2 metals react with Chlorine? |
form white solid chlorides |
|
|
How do GROUP 2 oxides react with water? Any exceptions? |
they react with water to form metal hydroxides which dissolve to form an alkaline solution due to the OH- ions
-Magnesium oxide is an exception as it reacts very slowly and the hydroxide is not very soluble. |
|
|
What happens to metal oxides as you go down the group in terms of its alkaline strength: |
the hydroxides get more soluble down the group so they get more alkaline down the group. |
|
|
What products are formed when group 2 oxides react with water? |
a hydroxide.
MO + 2H2O -----> M(OH)2 |
|
|
What products are formed when group 2 oxides react with acids? |
salt + water.
MO + HCL ------> MCl2 + H20 |
|
|
What products are formed when group 2 hydroxides react with water? |
metal + hydroxide ion (dissociation)
M(OH)2 -----> M2+ + 2OH- |
|
|
What products are formed when group 2 hydroxides react with acid? |
salt + water M(OH)2 + 2HCl ----> MCl2 + 2H2O |
|
|
Compare what happens to the solubility of a group 2 compound with anions that have a -1 charge vs. a -2 charge as you go down the group: |
For -2 anions (SO42-) compounds, solubility decreases down the group.
For -1 anions (OH-) compounds, solubility increases down the group. |
|
|
Which sulfate from Group 2 is known to be insoluble? Mg/Cl/Sr/Ba |
Barium Sulfate is insoluble in water. |
|
|
Define Thermal Decomposition: |
this is when a substance breaks down when heated. The more thermally stable a substance is, the more heat it requires to break down. |
|
|
What happens to thermal stability down the group for Carbonates and Nitrates ? |
It becomes more stable. This is because small cations cause more distortion than larger cations. This is due to charge density (the size of an ion in proportion to its charge). Because going down the group means a larger cation, there will be less distortion of the anion making it more thermally stable. |
|
|
Are Group 1 or Group 2 compounds more stable? |
Group 1 (because they have less charge therefore, less distortion!) |
|
|
What happens when Group 1 Carbonates and Nitrates are heated? |
Group 1 Carbonates: nothing -they're thermally stable unless you really heat them (except Li2CO3) ---> Li2O + co2).
Group 1 Nitrates: decompose to form a nitrite and oxygen.
Example: 2MNO3 ----> 2KNO2 + O2 |
|
|
What is the chemical way a Nitrite is written? |
-NO3 |
|
|
What occurs when Group 2 Nitrates and Carbonates are heated? |
Group 2 Carbonates: decompose to form an oxide and carbon dioxide.
Group 2 Nitrates: decompose to form an oxide nitrogen dioxide and oxygen |
|
|
How would you test how easily nitrates decompose? |
-how long it takes until oxygen is produced (relights glowing splint)
- how long until NO2 (brown toxic-smelling gas) is produced |
|
|
How would you test how easily Carbonates decompose? |
look at how long it takes for CO2 to be produced - test this with limewater .... should turn cloudy. |
|
|
How do you carry out a flame test? |
1) Clean a platinum or nichrome (a nickel-chromium alloy) wire by dipping it into concentrated hydrochloric acid and then holding it in a hot Bunsen flame. Repeat this until the wire doesn't produce any colour in the flame. 2) Dip the platinum/nichrome wire once more into HCL and then dip it into the sample that you are testing and hold it over the flame and note the colour produced. |
|
|
What colors to the following element give in a flame test?
-Li -Ca -Na -Sr -K -Ba -Rb -Cs |
Li --> red Na ---> yellow/orange K----> lilac Rb ---> red Cs---->blue Ca---->brick red Sr----->crimson Ba------>green |
|
|
Why do colour changes occur in flame tests: |
the energy absorbed from the flame causes particles to move to higher energy levels. As they fall down to lower energy levels , they release energy in the form of light. The difference in energy between the higher and lower levels determines the wavelength which determines its colour. |
|
|
Give the physical state and colour of each of the halogens: -F2 -CL2 -BR2 -I2 |
Flourine: colour ---> yellow state: gas Chlorine: colour ---> green state: gas Bromine: colour ---> red-brown state: liquid Iodine: colour ----> grey state: solid |
|
|
What happens to the electronegativity as you go up the group of the Halogens? |
it increases |
|
|
What would you say about the solubility of Halogens in water? |
they aren't very soluble in water |
|
|
What colours are Chlorine, Bromine and Iodine in water? |
Chlorine: virtually colourless Bromine: yellow/orange Iodine: brown |
|
|
What colors are Chlorine, Bromine and Iodine in Hexane (an organic solvent)? |
Chlorine: virtually colourless Bromine: orange/red Iodine: pink/violet |
|
|
What would you say about the solubility of the halogens in organic solvents? |
they dissolve very well in organic solvents due to the fact that they are covalently bonded (as they are diatomic). |
|
|
What happens to the Halogens in terms of reactivity and oxidizing ability as you go down the group? Explain... |
Halogen atoms react by gaining an electron in the outer p-subshell. When this occurs, they are reduced therefore they are oxidizing agents. As you go down the group, we know that the atoms get larger so the distance between the outermost electrons and the nucleus increases which decreases the electrostatic attraction between them and there is more shielding as well so it is harder for them to attract electrons making them less reactive and less oxidizing! |
|
|
What happens to the melting/boiling point of the halogens as you go down the group? Explain... |
it increases.
WHY? because halogens are diatomic gas molecules, the intermolecular forces that holds them together are Van Der Waals dispersion forces. Because the size of their atoms increase down the group, this means that the surface area of their atoms also increases so more temporary dipoles are able to form so more heat is needed to break these attractions. |
|
|
Define Disproportionation: |
these are reactions in which a substance is both oxidized and reduced at the same time. |
|
|
Do Halogens under disproportionation? If yes, is it mostly with alkalis or acids? |
Yes, with both cold and hot ALKALIS |
|
|
What is the oxidation number of the Cl- ion? |
-1 |
|
|
What is the oxidation number of chlorine (diatomic state)? |
0 |
|
|
What is the oxidation number of the ClO- (chlorate) ion? |
+1 |
|
|
What is the oxidation number of the BrO2- ion? |
+3 |
|
|
What is the oxidation number of the BrO- (bromate) ion? |
+1 |
|
|
What is the oxidation number of the IO3- ion? |
+5 |
|
|
What is the oxidation number of the IO4- ion? |
+7 |
|
|
Do halogens oxidise or reduce other substances? |
they oxidize other substances (but they themselves are reduced) |
|
|
Describe what occurs in each stage when fluorine, chlorine, bromine and iodine react with hot iron. (Hint: are halogens reducing or oxidizing agents): |
2Fe (s) + 3F2 (g)--------> 2FeF3 (s) (iron iii flouride) 2Fe + 3Cl2 -----> 2FeCl3 (iron iii chloride) 2Fe + 3Br2 ------> 2FeBr3 (iron iii and iron ii) weaker oxidizing agent. Fe + 2I2 ----------> FeI2 (only iron ii iodide)
|
|
|
What happens to the oxidizing ability of the halogens as you go down the group? |
it decreases. |
|
|
Write the equation for the oxidation of Iron: |
2Fe ------> 2Fe 3+ + 6e-
|
|
|
Write the equation for the oxidation of Chlorine: |
3Cl2 + 6e- --------> 6Cl- |
|
|
Write the equation for the reaction of Chlorine with Sulfur: |
S8 (S) + 4CL2 (g) ---------> 4S2Cl2 (l) |
|
|
What is the colour change in the solution when Fe 2+ is oxidized by a halogen into Fe 3+? |
from green -----------> to orange |
|
|
What happens to the reducing power of Halides are you go down the group? Explain.. |
the reducing power increases down the group. why? -the ions get bigger so it is easier to remove electrons since there is less attraction between the nucleus and outermost shell due to more distance. -more shielding |
|
|
What occurs when KF reacts with H2SO4? include state symbols |
KF(s) + H2S04(l) -------> KHS04(s) + HF(g) |
|
|
What occurs when KCL reacts with H2S04? include state symbols |
KCL(s) + H2S04(l) --------> KHS04 (s) + KCL (g) |
|
|
What occurs when KBr reacts with H2S04? include state symbols |
KBr (s) + H2SO4 (l) ----> KHSO4 (s) + HBr (g) further reduces.. H2S04 2HBr (aq) + H2SO4 (l) ----->Br2 (g) + SO2 (g) + 2H20 (l) |
|
|
What occurs when KI reacts with H2S04? include state symbols |
KI(s) + H2SO4 (l) -------> KHSO4 (s) + HI (g) further reduces H2S04 2HI(aq) + H2SO4 ------> I2 (g) + SO2 (g) + 2H2O which further reduces even more... S02 to H2S 6HI (g) + SO2 (g) -----> H2S (g) + 3I2 (s) + 2H20 (l) |
|
|
What colour are hydrogen halide gases? |
colourless |
|
|
Do hydrogen halides dissolve in water? |
yes, and they form strong acidic solutions |
|
|
How do hydrogen halides react with ammonia? |
they react to form white fumes |
|
|
How could you test the oxidizing strengths of the halide ions and what would their results be if you mixed each with potassium iodide? |
Mix with potassium iodide/chloride/bromide and displacement should occur. You can intensify the appearance by adding hexane (may appear vividly purple in case of iodine ions present) . Results: KI with potassium iodide ----> no reaction (will be purple if mixed with more reactive halide in hexane) KCl with potassium iodide ----> brown KB2 with potassium iodide -----> brown |
|
|
How to test for halides? And elaborate on each precipitate's solubility in ammonia... |
add nitric acid to remove impurities then add silver nitrate (AgNO3). These different colored precipitates can be used to identify they halide.
F- -------> no precipitate Cl- --------> white precipitate (soluble in dilute ammonia) Br- --------> cream precipitate (soluble in conc ammonia) I- -----------> yellow precipitate (insoluble in conc ammonia) |
|
|
How would you test for Silver halides? |
they should decompose in sunlight to form silver and a halogen! |
|
|
What two indicators are frequently used for titrations and what are their colors in alkali or acidic solutions? |
Phenolphthalein: pink in acid, colourless in alkali
Methyl Orange: yellow in acid, red in alkali |
|
|
How can you minimize uncertainties in equipment? |
-use more precise equipment -use larger volumes or larger weights to reduce uncertainty values
|
|
|
What are the typical uncertainty values of burettes and electronic scales? |
Burettes: around 0.1cm3 Scales: 0.005g uncertainty. |
|
|
What are systematic and random errors? |
Systematic: are the same every time you repeat the experiment which is caused by the set-up or the equipment.
Random: these are different each time you repeat the experiment done by the experimenter such as reading the burette wrong. |
|
|
How can you increase the reliability of your experiment? |
repeating it as many times as possible. (this cancels out random errors but not systematic) |
|
|
What are Iodine-Sodium Thiosulfate titrations useful for? |
they are useful for finding the concentration of an oxidizing agent. |
|
|
How would you carry out a Iodine-Thiosulfate titration ? |
We do this to figure out if something is an oxidizing agent. First, add the substance to acidified potassium iodide. It should oxidize some of iodide ions to iodine resulting in a brown solution if it is a true oxidizing agent. Stage 2: Next, we want to know the # of moles to see how concentrated the oxidizing agent is! So... we add the solution produced in stage 1 into a conical flask, then from a burette, add drop by drop the sodium thiosulfate and when it fades to pale yellow, add some starch to see if any of the iodine is still there. It should be dark blue. Keep adding until it becomes colorless showing that all of the iodine has been reacted with. This will show how many moles of iodine were in the solution! Then use this to hence, figure out the concentration of the actually oxidizing agent. |
|
|
List a few ways we could avoid making any random errors when carrying out our Iodine-Thiosulfate titration: |
-make sure that burette is not contaminated so wash with sodium thiosulfate. -read the burette correctly. -Wash flask between experiments -solutions may react with air so use them as soon as possible -only add the starch when the solution is pale yellow. |
|
|
What two things are necessary for a reaction to occur successfully ? |
-particles must be facing each other correctly
-collide with at least the minimum amount of kinetic energy. |
|
|
Define activation energy: |
this is the minimum amount of energy required for a reaction to occur where bonds are broken. |
|
|
Label and draw a Maxwell-Boltzmann Distribution showing the activation energy and the difference between high temperatures and low ones with the curve : |
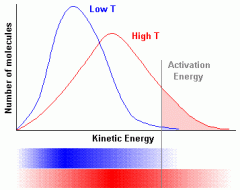
high temp: fatter, flatter and more to the right.
low temp: complete opposite |
|
|
Explain why increasing the temperature as shown by Maxwell-Boltzmann graphs show that the rate of the reaction increases and Describe what the shape of the graph will look like. |
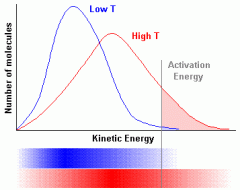
Once you increase the temperature, the kinetic energy of the particles increases so a greater proportion of the particles will have energy that is equal to or more than the activation energy causing the curve to shift more to the right. Also, increasing the temperature, increases the number of effective collisions which in turn, increases the rate of the reaction. |
|
|
Give five well-know things which will affect the rate of the reaction: |
-temperature -pressure -surface area -catalyst -concentration |
|
|
Why does increasing the concentration (or pressure) speed up the reaction? |
by increasing the temperature or pressure, we are causing the particles to become closer together as there are more present per unit volume. This increases their chances of colliding to give more effective collisions hence, increasing the rate of the reaction. |
|
|
Why does increasing the surface area speed up the reaction? |
if there is a larger exposed surface area, then more particles can collide with that substance per unit of time resulting in more effective collisions which speeds up the rate of the reaction. (We can increase the surface area of something by breaking it into smaller pieces) |
|
|
How does providing a catalyst speed up the reaction? |
catalysts lower the activation energy in a reaction by providing an alternative route (for bonds to be broken and made) in the reaction to occur. Also, if the activation energy is lower, it is more likely that particles will already have this energy, which increases the rate of the reaction. |
|
|
What is a homogenous catalyst? |
this is a catalyst which is in the same state as the reactants. |
|
|
How do homogenous catalysts speed up a reaction? What do their graphs look like? |
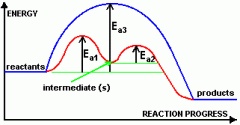
they speed up a reaction by forming one or more intermediate compounds with the reactants which form the products. The activation energy needed to form the intermediates is lower than needed to directly make the products from the reactants. Catalyst is also reformed again. |
|
|
Name two ways we can monitor the reaction rate based on the gas produced: |
-use a gas syringe to look at the volume of gas that has been produced. -use a balance and see how much decrease there is in the mass as the gas is evolved. |
|
|
What is the reaction between HCL and Sodium Thiosulfate solution and name one problem with it: |
hydrochloride acid + sodium thiosulfate ----> sodium chloride + sulfur dioxide + sulfur + water. it can be subjective though, as the yellow precipitate formed may not be agreed with by many people as being yellow at certain time frames. |
|
|
What is a dynamic equilibrium and what is needed to maintain it? |
this is when the forward reaction is equal to the backward reaction -it must be in a closed system in order to be maintained. |
|
|
What does Le Châtelier's principle state? |
if theres a change in the concentration, pressure, or temperature, the equilibrium will move to help counteract the change (by doing the opposite). -if temp is raised, it will attempt to cool it down -if pressure increased, it will attempt to decrease it |
|
|
What do catalysts affect and what do they not affect? |
they affect the rate of the reaction
they do not affect the position of the equilibrium. |
|
|
In a reversible reaction, which arrow points to the exothermic reaction and which arrow points to the endothermic one? |
right -------> exothermic (bonds are made this direction) left <-------- endothermic (bonds are broken this direction)
remember: BENDO MEXO (breaking bonds endo) (making exo) |
|
|
If you increase the temperature, where does the equilibrium shift? |
to the left. (the endothermic side) and vise versa |
|
|
If you increase the concentration, where does the equilibrium shift? |
to the side with less moles (and vise versa)
|
|
|
If you increase the pressure, where does the equilibrium shift? |
it shifts to the side with less moles of gas. (and vise versa) |
|
|
Hint: equilibriums If you were to place Iodine I chloride in your apparatus and you passed chlorine gas over it, what would occur and what colour will this substance be? If you pumped more chlorine in what would happen, and if more air is let in what would occur? |
iodine III chloride is formed (which is yellow)
ICl (l) + Cl2 (g) ⇌ ICl3
if you increase the concentration of chlorine, the amount of yellow product increases because the concentration of chlorine increases and it wants to produce less of Cl2. If you let more air in, the concentration of chlorine decreases so more ICL3 needs to decompose to form more chlorine. |
|
|
Suppose you had this colorless gas of N2O4 (dinitrogen teroxide) in a syringe and place this syringe in a water bath to increase the temperature, what colour would the gas in the syringe become. Why? Show the chemical reaction to help you! |
N2O4 (g) ⇌ 2NO2 (g) +58 kJ mol-1
increasing the temperature means that the endothermic (right side this time because its endothermic) will be favored so more NO2 brown gas will be produced causing it to turn from brown to dark brown. |
|
|
If you had this colorless gas of N2O4 (dinitrogen teroxide) in a syringe and pushed the syringe so the pressure would increase, what colour would the gas in the syringe become. Why? Show the chemical reaction to help you! |
N2O4 (g) ⇌ 2NO2 (g) +58 kJ mol-1
increasing the pressure means that the side with less moles would be favored (the right side) so more N2O2 (colorless) is produced so the mixture will go from brown to pale brown. |
|
|
When people are manufacturing chemicals in a business, what do they consider? |
- the maximum yield -the purity of the yield -the temperatures that need to be met (higher temperature means more expense) -the pressure required (more pressure means stronger pipes, hence more expense) -the amount of time required for a certain yield of the product to be produced. |
|
|
What does the term homologous series mean? |
anything that can be represented by the same general formula. (These substances also having similar properties). |
|
|
What is the general formula for alcohols? |
CnH2n+1OH |
|
|
How can you tell whether an alcohol is primary, secondary or tertiary? |
We base this upon how many alkyl groups the OH group is attached to. |
|
|
Which of the following is more reactive: Tertiary, Secondary, Primary? |
Tertiary (attached to more r-groups) |
|
|
Which one of these alcohols would be the easiest way to start off with making a halogenoalkane?
- primary alcohol -secondary alcohol -tertiary alcohol
and briefly describe how would you make chloroalkane - no need to describe purification process.
|
tertiary (as they are more reactive).
add the tertiary alcohol to hydrochloric acid to form the chloroalkane. -or mix phosphorus (v) chloride (PCl5) to an alcohol which form a chloroalkane + POCl3 (phosphoric chloride) + HCL. |
|
|
Being that bromoalkanes as well as iodoalkanes are difficult to make compared to chloroalkanes, using tertiary alcohols, what other alternative would you use to make it? Are there any disadvantages to using this process? |
For making bromoalkanes we won't use hydrogen bromide, we will use concentrated sulfuric acid (H2SO4) and a metal halide like KBr which will produce HBr which are both oxidized by the H2SO4 to form I2 which is a draw back as there will be a reduced yield; the bromoalkane is distilled off. For iodoalkanes, we repeat the same process but instead we use phosphoric (V) acid instead of sulfuric acid. - another alternative is reacting the alcohol with phosphorus (III) halide which produces an phosphoric acid (h3po3) and the halogenoalkane. |
|
|
How would you test for a hydroxyl group? |
add phosphorus (v) chloride to the unknown liquid. If the OH is present, steamy fumes of HCL should be produced which dissolve in water to form chloride ions. You can test for chloride ions using aqueous silver nitrate (and nitric acid to purify) and a white soluble precipitate forms. You can test the HCL by damp blue litmus paper turning red. |
|
|
What do alcohols react with Sodium to produce? |
Alkoxides |
|
|
What happens to the reactivity of the alcohol, the longer the hydrocarbon chain gets? |
it decreases because it becomes less soluble (more hydrocarbon "tails" in the way) |
|
|
Give the reaction for sodium reacting with ethanol: |
2CH3CH2OH + 2Na -----> 2CH3CH2ONa +H2 forms (this reaction is almost the same as reacting sodium with water except instead of the end product being NaOH + H2 the NaOh is in reverse so it looks like HONa + H2) |
|
|
What does water + metal give you? |
hydroxide + hydrogen |
|
|
Give one reason why you think alcohols have such a high boiling point: |
their OH groups can form hydrogen bonds. (These relate to hydrogen bonding which is the strongest intermolecular force) this gives it high melting points. |
|
|
Define "miscible" : |
miscible: If two liquids dissolve in each other they form a single continous layer (unlike water and oil which is immiscible) |
|
|
Are all alcohols miscible in water? why? |
No, though they can form hydrogen bonds in water, only short chain alcohols can do this (like methanol, ethanol, propan1-ol) otherwise if the alcohol's hydrocarbon chain is too large, their miscibility in water decreases (note: short chain alcohols can also dissolve in organic solvents like cyclohexane!). |
|
|
How colour do alcohols burn and what products do they form: |
they burn with a pale blue flame. The c-c bonds are also broken as the ethanol is oxidized to form CO2 and water.
so ROH + O2 ---------> CO2 + H2O |
|
|
What could you use to oxidize alcohols? |
Acidified Potassium Dichromate (VI) |
|
|
What are primary, secondary and tertiary alcohols oxidized to: |
Primary ------> aldehydes which oxidize to carboxylic acids Secondary------> ketones only Tertiary --------> nothing - won't be oxidized |
|
|
What is the formula for aldehydes and ketones, what is their functional group, what sort of compounds do they form and what do their end names sound like in compounds? |
CnH2nO functional group: C=O are carbonyl compounds
ketones: ends with "none" e.g propanone aldehydes: ends with "al" e.g propanal
|
|
|
Explain the procedure of how you would oxidize the primary alcohol ethanol to form an Aldehyde: |
1)gently heat ethanol with potassium dichromate (vi) solution with sulfuric acid with test tube with produces apple-smelling ethanal (aldehyde) to prevent further oxidization to form vinegar-smelling ethanoic aid, we should ensure that it is distilled as soon as possible using liebig (water in water out). |
|
|
Define the term volatile: |
used to describe a substance with a low boiling point that changes its state quickly |
|
|
Explain the procedure of how you would oxidize the primary alcohol like ethanol to form a Carboxylic Acid: |
1) alcohol must be vigorously oxidized (with excess potassium dichromate (vi)) past the aldehyde stage under reflux (increase the temperature of the organic reaction by heating without losing volatile solvents, reactants or products as they cool and condense back. The solution changes from orange to green during the formation of carboxylic acids. |
|
|
What chemical equation shows the oxidization of a primary alcohol to an aldehyde to a carboxylic acid? |
R-CH2-OH + [O] ---------> R-C=O + H2O + [O] ----> reflux---> R-C=O | | H OH |
|
|
How would you oxidize an alcohol to get a ketone? whats the equation? |
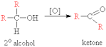
reflux a secondary alcohol with acidified dichromate (vi) which produces a ketone.
|
|
|
How would you oxidize an alcohol to get a ketone? whats the equation? |
tertiary alcohols can't be oxidized easily so nothing really happens - solution stays orange. |
|
|
How could you distinguish between Aldehydes and Ketones? |
Aldehydes: use Fehling's of Benedict's solution which are both Cu2+ complexes with reduce to form brick red Cu2O when warmed with aldehydes but stays blue with ketones. or - use Tollen's reagent which is reduced to silver when warmed with an aldehyde but not with a ketone to form a pretty silver mirror! |
|
|
How do you distinguish between primary, secondary, tertiary halogenoalkanes? |
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group. In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. |
|
|
What do you get when you mix a halogenoalkane with water? What's the chemical equation. |
R-X + H2O ----> ROH + H+ + X-
it forms an alcohol! |
|
|
How do you test for halides? |
add silver nitrate solution in the mixture which gives a silver halide precipitate. |
|
|
How could you demonstrate the reactivities of halogenoalkanes by showing and comparing the reactivities of primary, secondary and tertiary halogenoalkanes? |
Use 3 isomers of the same halogenoalkane and place into test tubes mixed with ethanol as a solvent and silver nitrate solution to indicate then place the test tube in a water bath at 50 degrees. The tertiary halogenoalkane will form first, then the secondary, then finally the primary which shows that tertiary halogenoalkane is the most reactive. |
|
|
What are the uses of halogenoalkanes? |
-can be used to make useful polymers such as (poly)chloroethene of PVC for pipes -(poly)tetrafluoroethene for non-stick pans -can be used for fridges as they don't corrode pipework -some are non-flammable used for flame retardants used in children's pajamas or plastic parts of computers. |
|
|
What are the risks of using halogenoalkanes? |
-chloroflourocarbons (all hydrogens replaced with cl and f) were discovered to be useful for fridges however they cause great damage to the ozone layer as the environmental problems outweighed the advantages so they were banned. -instead hydrochloroflourocarbons are used instead as they are less damaging, less stable and decompose lower in the atmosphere.
|
|
|
Why are carbon-halogen bonds polar? |
because the halogens are more electronegative than carbon |
|
|
What is a nucleophile? |
this is an electron-pair donor so it donates as electron pair to places without enough electrons. |
|
|
What are two examples of nucleophiles? |
-OH- -NH |
|
|
Show Nucleophilic substitution between halogens and nucleophiles: |
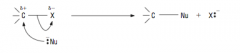
|
|
|
What is produces when a halogenoalkane is hydrolyzed? |
it turns into an alcohol |
|
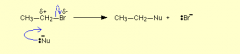
Complete the equation and Draw the nucleophilic substitution reaction for
CH3CH2Br + OH- ------------------> ???? |
CH3CH2Br + OH- ------------------> C2H5OH +Br- reflux the halogen is Br. The nucleophile is the OH which provides a pair of electrons to the C. The CBR bond breaks heterolytically and the halogen falls off.
|
|
|
Complete the equation and Draw the nucleophilic substitution reaction for
CH3CH2Br + H2O ------------------> ???? |
it will be much slower as water is a weaker nucleophile. CH3CH2Br + H2O ------------------> C2H5OH +Br- + H+
|
|
|
Complete the equation and Draw the nucleophilic substitution reaction for
CH3CH2Br + NH3 ------------------> ???? |
reflux CH3CH2BR+2NH3-------->CH3CH2NH2 +NH4BR ethanol |
|
|
How would you turn a halogenoalkane into an alkene? Use bromoethane as an example and make sure you know how to draw its mechanism: |
Use an elimination reaction so instead of having the halogenoalkane reacting with an aqueous alkali, it will be reacting with an alcoholic alkali instead! Make sure its also heated under reflux. ethanol CH3CH2BR +KOH------>CH2=CH2+H2O+KBR reflux |
|
|
What is an addition reaction? |
joining two or more molecules together to form a larger molecule |
|
|
What is an polymerization reaction? |
joining lots of simple molecules to form a giant molecule |
|
|
What is an elimination reaction? |
when a smaller group of atoms breaks away from a larger molecule |
|
|
What is a substitution reaction? |
when one species is replaced by another |
|
|
What is a hydrolysis reaction? |
it is the splitting of a molecule using either OH- or H+ derived from water. |
|
|
What is an oxidation reaction? |
any reaction in which an atom looses electrons |
|
|
What is a reduction reaction? |
any reaction in which an atom gains electrons |
|
|
What is a redox reaction? |
any reaction in which electrons are transferred between two species and both oxidation and reduction both occur. |
|
|
Describe the process of free-radical substitution when forming chloroalkanes: |
uv CH4 + Cl2CH3Cl + HCl stages: 1) Initiation (free-radicals made) sunlight provides energy to break the cl-cl bond through photodissociation: Cl2--->2Cl• 2)propagation (free radicals are used up in a chain reaction). CH4 + Cl•----->CH3• + HCl CH3• + Cl2------>CH3Cl + Cl•
|
|
|
CONT OF free-radical substitution of chloroalkanes: |
3)Termination (free radicals are used up to form a stable molecule): 2Cl•---->Cl2 CH3• + Cl•----->CH3Cl CH3• + CH3•------>CH3CH3
|
|
|
Describe the electrophilic addition of bromine to from bromoalkanes: |
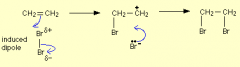
1)the double bond repels the electrons in Br2 polarising Br-Br. 2)heterolytic fission of Br2 this is where one Br gives up bonding electrons to the other Br and sticks to the c atom. 3)a positive carbocation intermediate is formed and the Br sticks to it as well. |
|
|
Describe the electrophilic addition of hydrogen bromide to from bromoalkanes: |
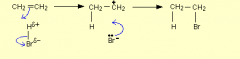
1)the molecule is already polar so it gets attracted to the double bond.2)heterolytic fission of HBr this is where one Br gives up bonding electrons to the H and sticks to the c atom. 3)a positive carbocation intermediate is formed and the Br sticks to it as well. |
|
|
What two products could possibly form when making hydrogen bromide in electrophilic addition reactions? WHY does this happen? |
2-bromopropane (major product as it has more r-groups)
or
1-bromopropane (smaller amount as it has less r-groups and is therefore less stable) |
|
|
What are electrophilic addition reactions? |
an electrophilic addition reaction is an addition reaction where, in achemical compound, a π bond is broken and two new σ bonds are formed - it usually involves alkenes. |
|
|
What are free-radical substitution reactions? |
These are reactions in which one atom in a molecule is replaced by another atom or group of atoms. Free radical substitution often involves breaking a carbon-hydrogen bond in alkanes |
|
|
What are nucleophiles? |
these are electron-pair donors. They are electron rich so they are attracted to places with a lack of electrons like polar bonds as they have slightly positive areas or even positive ions. |
|
|
What are electrophiles? |
these are electron-pair acceptors. They are electron-poor so they are attracted to places with more electrons like negative ions and electron rich areas around the carbon double bond. |
|
|
What are free-radicals? |
these have unpaired electrons and are very reactive. They react with anything positive, negative or neutral! |
|
|
What is homolytic fission? |
this is when a bond breaks evenly so one electron moves to each atom which forms two uncharged free radicals with an unpaired electron each. |
|
|
What is heterolytic fission? |
this is when a bond breaks unevenly so both elections move to one atom which will form a positively charged cation (an electrophile) and and a negatively charged anion (nucleophile) |
|
|
Where is the ozone layer and what does it contain? |
it is in a layer in the atmosphere called the stratosphere. It contains ozone molecules, 03 |
|
|
What responsibility does the ozone layer hold? |
it is responsible for removing the dangerous energy from UV radiation which can cause damage to our skin through sunburns and skin cancer. |
|
|
According to Chemistry how is the ozone formed? |
this occurs when the right amount of UV radiation is absorbed by an oxygen molecule; because of this, the oxygen molecule splits into separate atoms called free radicals. The free radicals then combine with other oxygen molecules to form ozone O3 molecules. O2 + hv ----> O• + O• -----> O2 + O• -----> O3
|
|
|
Can uv radiation reverse? What will it form this time? |
O3 + hv -----> O2 + O• |
|
|
How do concentrations of oxygen stay constant in the ozone? |
because the ozone layer is being constantly destroyed and replaced as UV radiation hits molecules, it actually has an equilibrium so concentrations remain constant.
O2 + O• ⇌ O3 |
|
|
What did researchers discover about the Ozone layer? How did they do this? |
In the 1970s, a team from the British Antarctic Survey found that the concentration of the ozone over Antarctica was very low. 15 years later it was even lower. They actually thought it was their instrument but they got new ones and it was still the same! The published their results. It was later discovered that there were actual holes in the ozone. which allow UV radiation to reach the Earth. |
|
|
Show the reaction and briefly describe what is contributing to most of the ozone layer being damaged: |
The CFCs are responsible for this as Chlorine free radicals are being formed when the CFCs are broken down by UV radiation. CCL3F(g) ----->CCL2F• (g) + Cl•(g) these free radicals are in fact, catalysts that react with the ozone layer to form an intermediate. (ClO• and an oxygen molecule) Cl• + O3 ----> O2 + ClO• ClO• + O3 -----> 2O2 + Cl• |
|
|
What are some advantages of using CFCs? |
-they are used in fire-extinguishers, propellants and in aerosols and coolant gases in fridges and medical inhalers but are now trying to find less environmentally-damaging alternatives. |
|
|
Besides CFCs what else damage the Ozone? How? Where do they come from? |
Nitric Oxide (NO•) free radicals do this.
they come from nitrogen dioxides emitted from cars and aircraft engines. They act as catalysts (like chlorine) to react with the ozone and form intermediates. NO• + O3 ----> O2 + NO2• NO2• + O3 -----> 2O2 + NO• |
|
|
Define sustainability: |
it is the ability to use resources meanwhile ensuring that the next generation will have enough resources to remain constantly supplied by not using up a lot of fossil fuels or emitting damaging and toxic chemicals into the environment. |
|
|
How can we ensure that our ways of living lead to a more sustainable future according to green chemistry? |
-use renewable raw materials (e.g from plants) -use renewable energy sources (bioethanol, solar power, wind) -use less energy in general (increase efficiency e.g using microwave radiation in pharmacies instead of conventional heating systems) -ensure chemicals used are non-toxic (don't use lead, foam, dry cleaners) -make sure products and waste are biodegradable or recyclable. |
|
|
Give examples of really toxic chemicals that are usually used, where we find them, and how we've created new ways of making more stable alternatives of these products: |
Lead: used in paint, petrol, electric components alternatives include: lead free-paint and using mixtures of copper, tin and silver in electrical components. Foams: fire extinguishers. They deplete the ozone layer Dry cleaners: used in chlorinate hydrocarbons but are carcinogenic - supercritical liquid CO2 used. |
|
|
What two things used in green chemistry are well known in increasing efficiency? |
Catalysts: by speeding up the reaction and increasing the rate by providing an alternative route for it to take place at a lower activation energy.
High atom economy: this is the proportion of reactant atoms that become part of the desired product. |
|
|
Describe how to production of ethanoic acid has changed over the years: |
1) ethanoic acid was first made on the industrial scale by the oxidation of butane or naphtha using conditions of 150-200 C at 40-50 atm pressure using a cobalt catalyst but the atom economy was low (about 35%). 2)In 1963 the company BASF came up with an alternative method using methanol and carbon monoxide: CH3OH + CO----> CH3COOH it has 100% atom economy but it needs a high temp (300C) at 700 atm using a cobalt iodide catalyst. |
|
|
cont of Describe how to production of ethanoic acid has changed over the years:
|
3)In 1970, Monosanto used the same reaction as BASF but using an interesting catalyst called rhodium iodide which only required 150-200 C and 30-60 atm and an improved yield of 98% and the methanol can be obtained from biomass. 4) the cativa process uses the same process using iridium iodide and there are fewer byproducts so it is more efficient. |
|
|
What is the difference between atom economy and percentage yield? |
% yield is all about the how much product you get compared to the theoretical maximum product you could achieve.
atom economy is is all about the number of atoms that go from your reactants into your products |
|
|
Describe the Greenhouse effect: |
1)the sun emits electronegative radiation which includes UV, IR and light radiation. 2) the radiation from the sun reaches the Earth's atmosphere which is absorbed by atmospheric gases (causing the Earth to heat up) and some is reflected back towards space. 3) various gases in the troposphere (lowest layer) absorb some IR radiation and re-emit it in all directions which is called the green-house effect. If we didn't have this we would die as the temp would be 30 degrees lower than normal.
|
|
|
Describe and give example of green house gases and how they keep the atmosphere warm: |
1) the main green house gases are water vapour, carbon monoxide and methane. 2) they absorb IR radiation that cause the bonds in the molecule to vibrate which passes onto other molecules causing the temp to rise. The greenhouse affect contributed by each gas depends on how much of this gas is present in the atmosphere and the amount of energy each molecule of this substance can absorb. |
|
|
How do cows and trees contribute to the greenhouse effect? |
- cows produce a lot of methane which is a greenhouse gas and absorbs a lot of heat -trees are being chopped down to make paper but the trees are responsible for using CO2 for photosynthesis but chopping them down means that there is more CO2 in the air which is a greenhouse gas and absorbs more heat. |
|
|
How can global warming be explained as natural causes of the Earth's climate? |
-changes in the Earth's orbit may have some effects on different ice-age cycles called ice ages (cold periods) and interglacials (warmer periods). - -sunspots every 11 years may also affect this. -volcanic eruptions and meteor impacts throwing lots of dust in the air cause global cooling. |
|
|
How are scientists claiming to measure global nowadays? |
-sampling air in unpolluted places like remote islands and average temp and co2 levels are going up. -monitoring sea water as it has become more acidic as more co2 dissolves forming carbonic acid. -they also claim that it is anthropogenic - humans are to blame!
|
|
|
Define the term carbon footprint: |
this is the amount of greenhouse gases something causes to be released. The more co2 released by something, the bigger its carbon footprint. |
|
|
Define the term carbon neutral: |
this are activities that have no overall carbon emission into the atmosphere. |
|
|
Give an example of something that is not carbon neutral and how it has contributed to the greenhouse effect? |
Petrol is not carbon neutral
-releases CO2 in the environment which was trapped in the Earth. |
|
|
Give an example of something that is carbon neutral and how it has contributed to the greenhouse effect? Give one disadvantage. |
Bioethanol is carbon neutral
-it is a substitute for petrol and is produced using the fermentation process using maize. However, it can still be argued that there are many carbon emissions from this process as making fertilizers and powering agricultural machinery. Dis: many undeveloped countries may attempt this to become richer but this means that they will be sacrificing their fields for energy and won't have enough to eat. |
|
|
Give an example of something that is carbon neutral and how it has contributed to the greenhouse effect? Give one disadvantage. |
Hydrogen gas is carbon neutral
can be used in modified engines or fuel cells. A fuel cell converts hydrogen and oxygen into water which produces electricity to power the vehicle and it renewable as hydrogen can be extracted from water using solar or wind power. Dis: many people consider hydrogen dangerous as it is flammable (like the Hindenburg incident). |
|
|
How does Infrared Spectroscopy work? |
1) a beam of IR radiation is passed through the sample. 2) the IR radiation is absorbed by their covalent bonds in the molecules increasing their vibrational energy. 3) bonds between different atoms absorb different frequencies of IR radiation. Also, the position of the bond absorb different frequencies (so the OH group in an alcohol compared to the OH group in a carboxylic acid absorb different frequencies) |
|
|
What sort of molecules can only be vibrated by IR radiation and explain why this occurs: |
the molecule must be made up of different atoms. Why? because automatically, if they are made up of different atoms, their polarities change as they vibrate. |
|
|
What can you say about gases that do absorb IR radiation? Why? |
they are greenhouse gases because they stop some of the radiation emitted by earth from escaping to space (so they must vibrate do do this) |
|
|
What are the certain fixed energy levels in gases and the limits to how much each IR radiation it can absorb called? |
Quantised levels. (this means that the energy that a gas can absorb is in 'levels' like a staircase; there are specific levels of steps one step to the next; you can't move 'half' a step up or a 'quarter' of a step up. Instead, the amount you go up is specified and standardized). |
|
|
When looking at equilibriums and analyzing whether increasing the pressure would shift the equilibrium to the left or to the right, what do you need to make sure about the reactants and products beforehand? |
ALL the reactants and products must be in their gaseous states in order to assess the effects of pressure. (Careful, they may trick you with this one on the exam!) |

