![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
143 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Mass Number |
Total number of protons and neutrons in the nucleus of an atom. |
|
|
|
Atomic Number |
Number of protons in the nucleus of an atom. |
|
|
|
Ions |
Ions have different number of protons and electrons. |
|
|
|
Isotopes |
Isotopes of an element are atoms with the same number of protons but a different number of neutrons. |
|
|
|
Bohr's model |
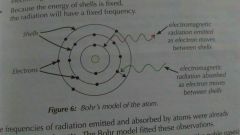
Electrons exist in shells, each shell has a fixed energy. When an electron moves between shells electromagnetic radiation is emitted or absorbed. |
|
|
|
Relative Atomic Mass |
The average weighted mass of an atom of an element compared to 1/12th of an atom of carbon-12. |
|
|
|
Relative Isotopic Mass |
The average weighted mass of an isotope of an element compared to 1/12th of an atom of carbon-12. |
|
|
|
Relative molecular mass |
The average weighted mass of a molecule compared to 1/12th of an atom of carbon-12. |
|
|
|
Relative Formula Mass |
The average mass of a formula unit, compared to 1/12th of an atom of carbon-12. |
|
|
|
Mole (definition) |
Amount of substance or 6.02x10^23 particles (the Avogadro constant) |
|
|
|
Moles of a substance (calculation) |
Mass of a substance / Molar mass |
|
|
|
Moles (from the number of atoms or molecules) |
Number of particles / number of particles in a mole (6.02x10^23) |
|
|
|
Molar mass |
The mass of one mole of something. |
|
|
|
Moles of a gas |
Volume in dm3 (cm3) / 24 (000) |
|
|
|
Ideal gas equation |

pV = nRT |
|
|
|
Moles of a solution |
(Concentration x Volume) / 1000 |
|
|
|
Empirical def. |
The smallest whole number ratio of atoms of each element in a compound. |
|
|
|
Molecular Formula |
The actual number of atoms of each element in a molecule |
|
|
|
How do you find the molecular formula from the empirical formula? |
1.) Find the empirical mass 2.) Divide molecular mass by the empirical mass 3.) Multiply empirical formula by that number |
|
|
|
Reaction Stoichiometry |
The ratios of reactants to products |
|
|
|
Nitrates molecular ion |
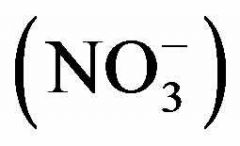
|
|
|
|
Carbonates molecular ion |
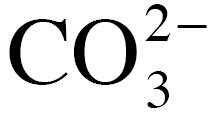
|
|
|
|
Sulfates molecular ion |
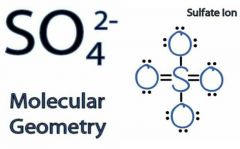
|
|
|
|
Hydroxide molecular ion |

|
|
|
|
Ammonium molecular ion |

|
|
|
|
Hydrochloric acid |

|
|
|
|
Sulfuric acid |
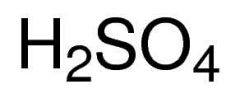
|
|
|
|
Nitric acid |

|
|
|
|
Sodium Hydroxide |

|
|
|
|
Potassium Hydroxide |

|
|
|
|
Ammonia |
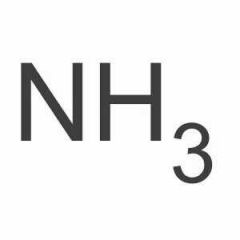
|
|
|
|
Strong acids |
Very little reverse reaction, nearly all the acid dissociates (ionise) |
|
|
|
Acid + Base |
Salt + Water |
|
|
|
Metal + Acid |
Salt + Hydrogen |
|
|
|
Metal Oxide + Acid |
Salt + Water |
|
|
|
Metal Hydroxide + Acid |
Salt + Water |
|
|
|
Metal Carbonate + Acid |
Salt + Water + Carbon Dioxide |
|
|
|
Hydrated salts |
A solid salt containing water of crystallisation |
|
|
|
Anhydrous salts |
A salt that doesn't contain water of crystallisation |
|
|
|
How do you find the formulas of hydrated salts? |
1.). Mass of anhydrous salt - mass of hydrated salt = mass of water 2.) Mass of water/18 = Moles of water 3.) Mass of anhydrous/Mr of anhydrous = Moles of anhydrous 4.) Divide both mole numbers by moles of anhydrous |
|
|
|
Methyl Orange |
Yellow to red when adding acid to alkali |
|
|
|
Phenophalein |
Pink to colourless when adding acid to alkali |
|
|
|
How do you prepare a standard solution? |
1.) Weigh out solid 2.) Transfer to a beaker, washing with distilled water 3.) Add water until completely dissolved. 4.) Transfer to volumetric flask, washing rod and beaker with distilled water 5.) Fill volumetric flask until bottom of meniscus reaches graduation line (use a pipette to add the last few drops) |
|
|
|
Percentage Yield = |
(Actual yield/theoretical yield) x 100 |
|
|
|
% Atom Economy = |
Mr of desired product/Mr of total products |
|
|
|
Addition reaction |
The reactants combine to form a single product. A + B -> AB |
|
|
|
Substitution Reaction |
Atoms from one reactant are swapped with atoms from another reactant. AB + CD -> AD + CB |
|
|
|
Advantages of high atoms economy in industry |
Less waste - costs less |
|
|
|
Oxidation Rules |
1.) Unconfined element = 0 2.) Ions have the same oxidation number as their charge 3.) Oxygen is always 2- unless told to work it out 4.) Hydrogen is always 1+ unless in a metal hydride where it is 1- |
|
|
|
Oxidising agents |
Accept electrons and gets reduced |
|
|
|
Reducing agents |
Donates electrons and get oxidised |
|
|
|
Redox reaction |
Reduction and oxidation happen simultaneously |
|
|
|
Shape of an s-orbital |
Spherical |
|
|
|
Shape of a p-orbital |
Dumbbell |
|
|
|
Maximum electrons in an s-subshell |
2 |
|
|
|
Maximum electrons in a p sub shell |
6 |
|
|
|
Maximum electrons in a d sub shell |
10 |
|
|
|
Maximum electrons in an f sub shell |
14 |
|
|
|
Maximum electrons the 1st shell |
2 |
|
|
|
Maximum electrons the 2nd shell |
8 |
|
|
|
Maximum electrons the 3rd shell |
18 |
|
|
|
Maximum electrons the 4th shell |
32 |
|
|
|
Ionic Bonding |
The electrostatic attraction between oppositely charge ions. Ionic crystals form a giant ionic lattice. |
|
|
|
Properties of ionic compounds |
Conduct electricity when molten or aqueous because the ions are free to move. Doesn't conduct when solid because ions are fixed in place. High melting and boiling point due to strong electrostatic forces that require a lot of energy to break. Ionic compounds are soluble |
|
|
|
Covalent Bonding |
Shared pair of electrons |
|
|
|
Dative Covalent Bonding |
One atom provides both of the shared electrons |
|
|
|
Electron Repulsion |
Lone pairs of electrons repel more than bonding pairs of electrons |
|
|
|
Tetrahedral |
Bonding pairs 4 Lone pairs 0 Bond angle 109.5° Example CH4 |
|
|
|
Linear |
Bonding pairs 2Lone pairs 0Bond angle 180°Example BeCl 2 |
|
|
|
Non-linear |
Bonding pairs 2Lone pairs 2Bond angle 104.5°Example H2O |
|
|
|
Trigonal Planar |
Bonding pairs 3Lone pairs 0Bond angle 120°Example BeF3 |
|
|
|
Trigonal Pyramidal |
Bonding pairs 3Lone pairs 1Bond angle 107°Example NH3 |
|
|
|
Trigonal Bipyramidal |
Bonding pairs 5Lone pairs 0Bond angle 120° and 90°Example PCl 5 |
|
|
|
Octahedral |
Bonding pairs 6Lone pairs 0Bond angle 90°Example SF6 |
|
|
|
Electronegativity |
The ability of an atom to attract the bonding electrons in a covalent bond |
|
|
|
Non - Polar bonds |
Atoms have equal electronegativity |
|
|
|
Polar Bond |
Atoms have different electronegativity |
|
|
|
Polar molecule |
Asymmetrical |
|
|
|
Non polar molecule |
Symmetrical |
|
|
|
Permanent dipole-dipole interaction |
The slightly - and slightly + charges on a polar molecule cause weak electrostatic attraction between molecules |
|
|
|
Induced Dipole-Dipole Interactions |
An uneven distribution of electrons in the electron charge cloud causes a temporary dipole which induces another temporary dipole in a neighbouring molecule - domino effect |
|
|
|
Hydrogen Bonding |

Only occurs when hydrogen is bonded to oxygen, nitrogen or fluorine. A weak bond from between the H on one molecule and the lone pair on another molecule |
|
|
|
Properties of simple covalent compounds |
Low melting and boiling point due to weak intermolecular forces, do not conduct electricity and are insoluble. Molecule containing H-bonding have a significantly high boiling point |
|
|
|
First Ionisation Energy |
The energy needed to remove 1 mole of electrons from 1 mole of gaseous atoms |
|
|
|
Factors affecting ionisation energy |
1.) Nuclear charge - the more protons, the more positive the charge, the stronger the attraction. 2.) Atomic Radius - An electron close to the nuclear is more attracted than one further away. 3.) Shielding - more electrons between the out electron and the nucleus, the less attraction |
|
|
|
Trends in ionisation energy down a group |
Generally fall due to extra shell, so the atomic radius increases and reduces attraction |
|
|
|
Trends in ionisation energy across a period |
Ionisation energy generally increases because there are more protons, so a higher nuclear charge and a smaller atomic radius. |
|
|
|
Drop in ionisation energy between Group 2 and 3 |
caused by outer electron in group 3 being in a p orbital rather than an s orbital. |
|
|
|
Drop in ionisation energy between Group 5 and 6 |
Due to electron repulsion |
|
|
|
Successive Ionisation Energy |
The energy need to remove 1 mole of electrons from 1 mole of gaseous ions |
|
|
|
Trends in Successive Ionisation Energy |
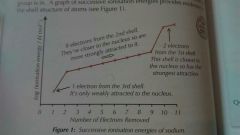
Successive ionisation energy increases due to electrons being remove from an increasingly positive ion - less repulsion between remaining electrons. There is a big jump when a new shell is broken into. |
|
|
|
Graphite |
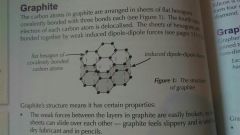
Carbon atoms arranged in sheets bonded to 3 other carbons and a delocalised electron. Sheets held together by induced dipole-dipole forces. Properties: can conduct electricity, a good lubricant, low density, high melting point, insoluble. |
|
|
|
Diamond |
Carbon atoms bonded to 4 other carbons, tetrahedral shape. Properties: very high melting point, extremely hard, good thermal conduct, insoluble.
|
|
|
|
Silicon |
Forms a crystal lattice structure like diamond with similar properties. |
|
|
|
Graphene |
One layer of graphite, a two-dimensional shape, good electrical conductor, extremely strong,incredibly light. Used in high speed electronics and aircraft technology. |
|
|
|
Metallic Bonding |
The electrostatic attraction between positive metal cations and delocalised electrons. |

|
|
|
Properties of metals |
High melting point due to strong electrostatic attraction. Malleable. Good electrical conductor. Insoluble. |
|
|
|
Group 2 |
Reactivity decreases down the group because there is less shielding and a small atomic radius so it is hard to lose the outer electrons. Used to neutralize acidic soil and excess stomach acid. |
|
|
|
Colour and state of Fluorine |
Pale yellow gas |
|
|
|
Colour and state of Chlorine |
Green gas |
|
|
|
Colour and state of Bromine |
Red-brown liquid |
|
|
|
Colour and state of iodine |
Grey solid |
|
|
|
Group 7 Reactivity |
Reactivity decreases down the group because there is more electron shielding and a bigger atomic radius so there is less nuclear attraction. Melting and boiling point increase down the group. Volatility decreases down the group. |
|
|
|
Group 7 displacement |
Fluorine displaces Chlorine. Chlorine displaces Bromine. Bromine displaces Iodine. |
|
|
|
Chlorine water |
Colourless |
|
|
|
Bromine water |
Orange |
|
|
|
Iodine water |
Brown |
|
|
|
Bromine water and potassium iodide |
Solution goes orange to brown |
|
|
|
Chlorine water and potassium bromide |
Solution goes from colourless to orange |
|
|
|
Addition of hexane to halogens |
Violet/pink - iodine Orange/red - bromine Pale yellow/green - chlorine |
|
|
|
Test for Halides |
Add silver nitrate and a few drops of dilute nitric acid. Chlorine - white precipitate, dissolves in dilute ammonia Bromine - cream precipitate, dissolves in concentrated ammonia Iodine - yellow precipitate, doesn't dissolve in concentration or dilute ammonia. |
|
|
|
Disproportionation reactions |
A single element is simultaneously reduced and oxidised |
|
|
|
Bleach |
Chlorine gas reacts with aqueous sodium hydroxide to form chloric acid (bleach) and water |
|
|
|
Enthalpy Change |
The heat energy transferred in a reaction at constant pressure |
|
|
|
Standard conditions |
100kPa and 298°K (25℃) |
|
|
|
Standard Enthalpy change of reaction |
The enthalpy change when a reaction occurs in the molar quantities under standard conditions |
|
|
|
Standard enthalpy change of formation |
The enthalpy change when one mole of a compound is formed from its elements in their standard states, under standard conditions |
|
|
|
Standard enthalpy change of combustion |
The enthalpy change when one mole of a substance is completely burned in oxygen under standard conditions |
|
|
|
Standard enthalpy change of neutralisation |
The enthalpy change when solutions of an acid and an alkali react to form one mole of water under standard conditions |
|
|
|
Exothermic reactions |
Give out energy to their surroundings - products have less energy than reactants. Will have a negative enthalpy change of reaction. |
|
|
|
Endothermic reactions |
Take in energy from surroundings - products have more energy than reactants. Enthalpy change is positive. |
|
|
|
Activation energy |
The minimum amount of energy needed to begin breaking reactant bonds and start a chemical reaction. |
|
|
|
Bond dissociation energy |
The amount of energy you needed per mole to break the attraction in a bond |
|
|
|
Bond breaking |
Exothermic |
|
|
|
Bond making |
Endothermic |
|
|
|
Enthalpy change of reaction = |
Total energy absorbed - total energy released |
|
|
|
Equation for enthalpy change |

q = mc🔺T |
|
|
|
Calculating standard enthalpy change of combustion |
1.) q = mc🔺T 2.) n = mass / mr 3.) 🔺c H = q / n |
|
|
|
Calculating standard enthalpy change of reaction |
1.) q = mc🔺T 2.) n = mass / Mr 3.) 🔺r H = q / n ( x number of moles reacting in a balanced equation) |
|
|
|
Hess's Law |
The total enthalpy change of a reaction is always the same, no matter which route is taken. |
|
|
|
Collision theory |
A reaction won't take place unless two particles collide with the minimum amount of Kinect energy needed. |
|
|
|
Enthalpy Profile Diagram |
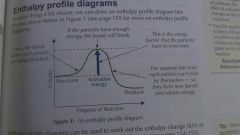
|
|
|
|
Boltzmann distributions |
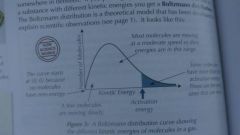
|
|
|
|
The effect of temperature on rate of reaction |
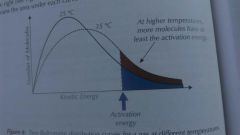
Increased temperature mean molecules have more kinetic energy so more molecule will have at least the activation energy. |
|
|
|
The effect of concentration on rate of reaction |

Increased concentration means more molecules in a given volume so a higher chance of collisions |
|
|
|
Catalyst |

Increase the rate of reaction by lowering the activation energy |
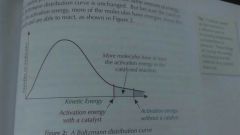
|
|
|
Heterogeneous catalyst |
In a different phase from the reactants. Example - Haber Process uses a solid iron catalyst |
|
|
|
Homogeneous catalyst |
In the same physical state as the reactants |
|
|
|
Catalytic converters |
Reduce the pollution released into the atmosphere by speeding up the reaction 2CO + 2NO --> 2CO2 + N2 |
|
|
|
Rate of reaction = |
Amount of reactants used or products formed / time |
|
|
|
Dynamic Equilibrium |
Where the forward and backward reaction occur at the same rate |
|
|
|
Le Chatelier Principal |
If there is a change in concentration, pressure or temperature the equilibrium will move to help counteract the change. |
|
|
|
Equilibrium: change in concentration |
Increase the concentration of reactants, equilibrium shift right to make more product. |
|

