![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
What is the formula for: Percentage Yield of a Chemical Reaction? |

MASS, not MOLES |
|
|
What is the formula for: Moles is a solution? |
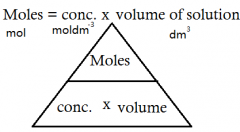
|
|
|
What is the Avogadro's constant? |
The number of particles in 1 mole of a substance ( 6.02 * 10 ^ 23 ) |
|
|
What is the formula for: The number of moles in a specified number of particles of a substance |
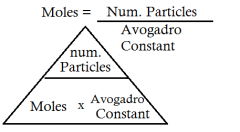
|
|
|
What is the formula for: The mass of a certain number of moles of a substance |
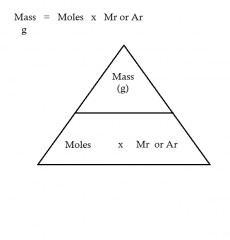
|
|
|
What is the definition of a mole? |
1 mole of a substance is the amount of substance with the same number of particles as 12 grams of carbon - 12 |
|
|
What are the usual charges of Ions in each group?
|
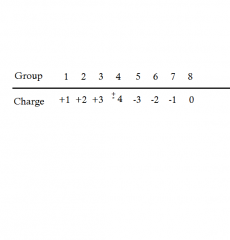
|
|
|
What are the ions and charges of Ammonium and Hydroxide? |
Ammonium = NH4 + Hydroxide = OH - |
|
|
What are the ions and charges of Sulfide and Sulfate?
|
Sulfide = S 2- Sulfate = SO4 2- |
|
|
What are the Ions and charges of Nitride and Nitrate?
|
Nitride = N 3- Nitrate = NO3 - |
|
|
What are the Ions and charges of Carbonate and Phosphate?
|
Carbonate = CO3 2- Phosphate = PO4 3- |
|
|
What are the charges of Iron(II) and Iron (III)
|
Iron(II) = 2+ Iron(III) = 3+ The number in the bracket tells you the charge. |
|
|
What are the formulas for the covalent compounds Hydrochloric Acid and Sulphuric Acid?
|
Hydrochloric Acid = HCl Sulphuric Acid = H2SO4 |
|
|
What are the formulas for the covalent compounds Nitric Acid and Phosphoric Acid?
|
Nitric Acid = HNO3 Phosphoric Acid = H3PO4 |
|
|
What are the products when you react: 1. Acid + Metal 2. Acid + Metal Oxide |
1. Acid + Metal --> Salt + Hydrogen 2. Acid + Metal Oxide --> Salt + Water |
|
|
What are the products when you react: 1. Acid + Metal Hydroxide 2. Acid + Metal Carbonate |
1. Acid + Metal Hydroxide --> Salt + Water and 2. Acid + Metal Carbonate --> Salt + Water + Carbon Dioxide |
|
|
What are the products when you react: 1. Acid + Hydrogen Carbonate 2. Acid + Ammonia |
1. Acid + Hydrogen Carbonate --> Salt + Water + Carbon Dioxide 2. Acid + Ammonia --> Ammonium Salt |
|
|
What are the products when you react: 1. Metal + Oxygen 2. Metal Carbonate, Heated (Thermal Decomposition) |
1. Metal + Oxygen --> Metal Oxide 2. Metal Carbonate -Heat-> Metal Oxide + Carbon Dioxide |
|
|
How do you convert cm^3 into dm^3? |
dm^3 = cm^3 / 1000 |
|
|
How many decimal places do you record the burette readings to and how do you decide which results are concordant? |
2 decimal places Results which are within 0.10cm3 of each other are concordant |
|
|
How do you write an ionic equation?
|
Split up aqueous ionic compounds into separate ions and cancel out the 'spectator' ions that do not change |
|
|
What number of significant figures should you use in your answers?
|
The same number of significant figures as the data value with the fewest significant figures |
|
|
How many drops of indicator should be added in a titration and why?
|
3 or 4 drops to make sure the indicator can be seen. No more than this because indicators are weak acids, will change the end point. |
|
|
Why should you always overfill the burette and then let some of the solution run out?
|
The area below the tap (the jet) is counted as part of the volume of the burette. So this has to be filled or the titre value will be higher than it should be. The air bubble |
|
|
What is a Standard Solution? |
A Solution whose concentration is known accurately. |
|
|
What is the formula for: Apparatus error for each piece of equipment? |
Margin of error ________________ x 100 Quantity Measured |
|
|
What is the formula for the ideal gas equation?
|

|
|
|
How do you convert from kPA (Kilopascals) to Pa(Pascals)?
|
x 1000 times by 1000
|
|
|
How do you convert from dm^3 (Decimeters cubed) to m^3 (Meters cubed)?
|
/ 1000 divide by 1000
|
|
|
How do you convert from cm^3 (Centimeters cubed) to m^3 (Meters cubed)?
|
/ 1.000.000 divide by A MILLION
|
|
|
How do you go from degrees Celsius to kelvin?
|
+ 273 add 273
|
|
|
What is the definition of atom economy?
|
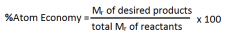
|
|
|
How can atom economy usually be improved?
|
By finding a use for the other byproducs e.g selling them or using them in other reactions.
|
|
|
What is the formula for working out the apparatus error for each piece of equipment?
|
Margin of error _________________ x 100 Quantity Measured |
|
|
What is the Empirical Formula?
|
The simplest whole number ratio of atoms of each element in a compound |
|
|
What is the Molecular Formula?
|
Actual number of atoms of each element in a compound
|
|
|
How would you work out the empirical formula when given a percentage of one element that a molecule contains? |
Divide the percentage by the Relative Atomic Mass of the element to work out the moles of each atom REMEMBER THIS. |
|
|
What indicators may be used in a titration and what colour change will occur at the end point?
|
Phenolphthalein. Colourless in acid. Pale pink at end point. Pink in alkali. Methyl Orange. Red in Acid. Pale orange at End point. Yellow in alkali. |
|
|
How would you calculate the Molecular Formula from an Empirical Formula? |
Compare the Mr of the Empirical with the Actual.
Then multiply to make the empirical match the actual. |
|
|
Outline the process for carrying out a Titration |
1. Clean Glassware with deionised water. Give Burette a wash with Standard Solution then fill it. 2. Let some solution out into waste beaker to fill the area below tap (Jet). If not done, titre volumes will be too high. 3. Rinse Pipette with unknown solution before pipetting 25cm3 into a conical flask. 4. Add 3 or 4 drops of indicator to conical flask and place under burette. 5. Continually swirl in a circular motion and slowly use burette tap to add a slow trickle of standard solution. Towards end point wash sides with deionised water to get all reactants in the solution reacting. Very slowly towards end point. 6. Mark down burette reading to 2 Decimal Places. 7. Wash conical flask with deionised water and go again. |
|
|
Why would a rough titration be carried out on an unknown solution? |
To determine the approximate amount of standard solution required. |
|
|
Why is a Standard Solution required in a Titration? |
Knowing the Concentration and Volume of Standard Solution accurately allows the concentration of unknown solution to be determined accurately. |
|
|
What would be different about subsequent titrations?
|
Add 2cm3 less than titre value to conical flask of standard solution then add dropeise from burette swirling. Saves time.
|
|
|
Outline the Process of Making a Standard Solution
|
1. Take weighing boat and place on balance. Tare Balance. Carefully add mass of substance and record mass to required number of decimal places.
2.Transfer substance to a beaker. Add deionised water to dissolve it. Rinse weighing boat at least twice in to beaker with deionised water to get all substance in beaker. 3. Stir with a stirring rod until all solid dissolves. 4. Transfer Solution from beaker to a Volumetric Flask using a funnel. 5. Rinse the Beaker, Glass rod and Funnel at least twice into volumetric flask to wash all substance into Volumetric flask. 6. Add deionised water until water reaches gradiations on the flask, add final drops with a teat pipette to ensure that Bottom of Miniscus is on the graduation mark. 7. Put lid on flask and invert flask several times to ensure solutions is homogenous and thoroughly mixed. 8. Label Solution with your Name, Date and Contents / Concentration. |
|
|
What are the units for the Ideal Gas Equation?
|
pV = nRT p = Pressure, Pa. Pascals V = Volume, M^3 Metres Cubed n = Mols R = Gas Constant (8.31JKMol-1) T = Temperature, Kelvin |
|
|
How could you test to see if a sample was completely dry? |
Take mass, heat to a temperature that water would evaporate. Take mass again, check that mass is unchanged. |

