![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Explain the terms - arene - aromatic |
Aromatic - a compound which contains at least one benzene ring. Arene - an aromatic hydrocarbon containing one or more benzene rings. |
|
|
Compare the Kekule and delocalised models for benzene Review the evidence for a delocalised model of benzene` |
Each carbon on the benzene ring is involved in three sigma bonds, one with each adjacent carbon and one with the hydrogen attached to the atom. This leaves one unpaired electron in a p-orbital on each carbon atom. The Kekule structure proposes that the six p-orbital electrons in the benzene ring formed 3 pi double bonds on alternating carbons The delocalised model suggests that these six electrons form pi bonds through the overlapping of each p-orbital with the two adjacent p-orbitals, such that the electrons become delocalised in the benzene ring and are no longer tied to any one carbon atom. Instead, a ring of electron density is formed above and below the plane of the carbon atoms. This means that the benzene ring is more stable than it would be according to the Kekule structure, as the delocalised electrons are as far apart as possible so there is minimum repulsion and maximum stability. There are several reasons as to why the delocalised model is now the generally accepted model of benzene: - If benzene did contain double bonds (i.e unsaturated), it should readily undergo addition reactions, and react similarly to alkenes e.g should decolourise bromine water. Benzene does not, in fact, undergo addition reactions as this would require delcoalised electrons to take part in bonding to the additional group, meaning the product would be less stable than benzene - this would not be energetically favourable. However, benzene undergoes electrophilic substitution as this allows the pi electrons to retain there delocalisation (only with permanently polar/charged molecules as its delocalised structure means it cannot induce polarity). - As C=C double bonds and C-C single bonds have different bond lengths, it would be expected, according to the Kekule structure, that benzene would contain two different bond lengths between alternating carbon atoms. However, it was found by Lonsdale that all of the bond lengths in the benzene ring are identical and of a length midway between that of a C-C bond and a C=C bond. - The enthalpy change that occurs when cyclohexene (with one double bond) undergoes hydrogenation is -120klmol-1. Therefore, if the Kekule structure of benzene is correct, the enthalpy change of hydrogenation of benzene (with three double bonds) should be -360kjmol-1. However, the actual value was found to be 208kjmol-1, showing that benzene is more stable than the proposed Kekule structure would suggest. the enthalpy change is 152kjmol-1 less, which is known as the delocalisation energy. - The enthalpy change of atomisation of benzene according to the kekule structure should be +5355klmol-1, however the experimental/actual value was found to be +5561kjmol-1, showing that benzene is harder to atomise than the Kekule model would suggest. - In accordance with the Kekule structure, there should be should be two different isomers, depending on where the double bonds are positioned within the ring. However, in practice, only one isomer is found. |
|
|
Describe the electrophilic substitution of arenes with concentrated nitric acid Outline the mechanism of electronphilic substitution of arenes |
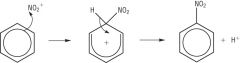
In benzene, the region of high electron density above and below the plane of carbon atoms attracts electrophiles (electron pair acceptors). The nitro group first has to be generated by reacting concentrated nitric acid with concentrated sulphuric acid: HNO3 + H2SO4 --> NO2+ + HSO4- +H2O The conditions for the reaction are: - concentrated acids (nitric and sulphuric) - temperature of 50-60 degrees celcius - reflux Overall equation: C6H6 + HNO3 --> C6H5NO2 + H2O H2SO4 is reformed by the reaction of the H+ ion with the HSO4 ion, therefore it acts as a catalyst to the reaction. Uses of nitrobenzene: dyes, pharmaceuticals, pesticides. |
|
|
Describe the electrophilic substitution of arenes with halogens |
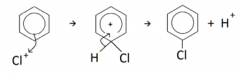
The halogen ion must first be generated from the reaction of a halogen molecule with a halogen carrier (e.g FeX3, AlX3) e.g Cl2 + AlCl3 --> Cl+ + AlCl4- The conditions for the reaction are: - Halogen carrier - Room temperature and pressure Overall equation: C6H6 + Cl2 --> C6H5Cl + HCl Uses: bromobenzene used in preparation of pharmaceuticals |
|
|
Explain the relative resistance to bromination of benzene compared with alkenes |
Alkenes with bromine: When an alkene is added to bromine water, it changes from orange to colourless. The alkene contains a C=C pi-bond which constitutes a region of high electron density, which polarises an approaching bromine molecule as the pi-bond electrons repel the electrons in the Br-Br bond. The closest bromine atom in the molecule becomes positive and the electron in the C=C double bond are attracted to it causing the double bond to break and a new covalent bond to be formed with the bromine atom. The Br-Br bond breaks by heterolytic fission, leaving a negative bromine ion which is attracted by the now positive carbon atom (carbocation) i.e the adjacent carbon to that now bonded to the first bromine atom, forming a new covalent bond. Benzene with bromine: When benzene is added to bromine water, the solution remains orange - no reaction takes place. If iron bromine (halogen carrier) is added, the mixture turns colourless and hydrogen bromide fumes are given off. The delocalised structure of benzene means that there is insufficient electron density on any specific carbon atom to induce polarity in an approaching bromine molecule. A halogen carrier generates a positively charged bromine ion which is able to attract the delocalised pi-electrons in the benzene ring so that the reaction can take place. |
|
|
Describe the reactions of phenol with aqueous alkalis and with sodium |
Phenols - a class of organic compounds in which a hydroxyl (-OH) group is directly attached to a benzene ring. Aqueous alkalis - e.g sodium hydroxide Phenol dissolves in water to form a weak acidic solution by losing an H+ ion from the -OH group C6H5OH + H2O --> C6H5O- + H+ Therefore, phenol can be neutralised by aqueous sodium hydroxide : C6H5OH + Na+[C6H5O-] + H2O Sodium C6H5OH + 2Na --> 2Na+[C6H5O-] + H2 The product of both reactions is the salt sodium phenoxide (contains ionic bonding) |
|
|
Describe the reaction of phenol with bromine |
Phenol reacts with bromine without a halogen carrier. The reaction takes place at room temperature. C6H5OH + 3Br2 --> C6H2Br3OH + 3HBr This product is called 2,4,6 - tribromophenol and it precipitates out of solution after turning bromine water colourless.
|
|
|
Explain the relative ease of bromination of phenol compared with benzene |
The increased reactivity of phenol occurs because the lone pair of electrons of the oxygen of the -OH group is drawn in towards the benzene ring, creating a higher electron density of the ring structure. This activates the benzene ring, such that it is able to polarise the bromine molecules so that the reaction may take place without the use of a halogen carrier. |
|
|
State the uses of phenols in plastics, antiseptics, disinfectants and paints. |
Alkyl phenols are used in detergents Chlorophenols are used in antiseptics and detergents Salicyclic acid (phenol with carboxylic acid group) if used in preparation of aspirin and other pharmaceuticals Bisphenol is used in production of epoxy resin for paint. |
|
|
Describe the reactions involved in the production of phenol from nitrated benzene |
![Nitrated benzene is reduced to form aminobenzene:
C6H5NO2 + 6[H+] --> C6H5NH2 + 2H2O
Conditions:
- reflux
- tin
- concentrated HCl
Aminobenzene undergoes diazotization to a diazonium ion
C6H5NH2 + HNO2 + HCl --> Cl-[C6H5N2+] + 2H2O
HNO2 ...](https://images.cram.com/images/upload-flashcards/49/54/97/12495497_m.png)
Nitrated benzene is reduced to form aminobenzene: C6H5NO2 + 6[H+] --> C6H5NH2 + 2H2O Conditions: - reflux - tin - concentrated HCl Aminobenzene undergoes diazotization to a diazonium ion C6H5NH2 + HNO2 + HCl --> Cl-[C6H5N2+] + 2H2O HNO2 (nitrous acid) has to first be made in situ: NaNO2 + HCl --> HNO2 + NaCl Conditions: - temerature <10 degrees celcius Benzene diazonium chloride undergoes hydrolysis (substitution reaction) to form phenol Cl-[C6H5N2+] + H2O --> C6H5OH + HCl + N2 Conditions: - temperature > 10 degrees celcius |

