![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
What is the digestive system made up of & what is the process of digestive? |
*Its made up of a large muscular tube & its associated glands. *Digestion is the process in which large molecules are hydrolysed by enzymes to produce smaller molecules that can be absorbed & assimilated. *The digestive system provides an interface with the environment.
|
|
|
What are the 2 stages of digestion & explain the 3 components of saliva? |
*Physical breakdown - Mastication of food uses the teeth to increase the surface area. *Chemical digestion - Is the use of enzymes to break down large, insoluble molecules into smaller, soluble ones. *Salivary amylase hydrolyses starch into maltose. *Mucin {mucus} binds food into bolus lubricted for swallowing. *Alkali neutralises any acid in food. |
|
|
How does the oesophagus assist digestion? |
*Bolus is pushed back by tongue to the back of the mouth. *The oesophagus is a long tube made up of a thick muscular wall. *Epiglottis prevents food from entering the trachea. *Mucus is secreted from glands to lubricate food. *Peristalsis pushes bolus to stomach. |
|
|
How does the stomach aid digestion? |
*The stomach is a muscular, holds approximately 1.2 litres & increase to 5 litres when distended. *Converts food into semi solid chyme by peristaltic churning. *Gastric mucosa embedded with gastric pits that produce gastric juice. *Gastric juice contains:- - Water - Hydrochloric acid -> creates pH2 which kills bacteria -> denatures amylase. - Pepsin -> digests proteins & amylase. *Chyme is gradually released over 3 to 4 hours. *Pyloric sphincter controls exit in small squirts. |
|
|
How is the small intestine adapted to its function & what are the 3 digestive juices in the small intestine? |
*Over 6 metres long. *Luminal epithelium is folded into villi & microvilli to increase surface area so the small intestine is in contact with more food. *Maintains concentration gradient - Peristalsis delivers more nutrients, active transport removes nutrients from lumen & extensive capillary network provides an excellent blood supply to remove nutrients from epithelial cells. *Short distances - 1 cell thick epithelium, capillaries lie next to epithelial cells & thin walls surround capillaries. *Bile, pancreatic juice & intestinal juice. |
|
|
What is bile & pancreatic juice? |
*Bile - Created by the liver, stored in gull bladder, secreted by bile duct into small intestine, contains alkali -> neutralises acidic chyme for enzymes. *Pancreatic juice - secreted by pancreas via pancreatic duct, is alkaline -> neutralises stomach acid - optimum pH & contains various enzymes e.g. pancreatic amylase -> hydrolysis of starch into maltose. |
|
|
What is intestinal juice & what are membrane - bound enzymes? |
*Intestinal juice - secreted from cells covering villi, contains alkali & mucus & may contain few, if any enzymes. *Membrane - bound enzymes - bound to luminal membrane of epithelial cells, may be found in intestinal juice due to sloughing of these cells. Carbohydrases - hydrolyse glycosidic bonds, releasing monosaccharides:- - Sucrase digests sucrose -> glucose + fructose - Maltase digests maltose -> glucose - Lactase digests lactose -> glucose + galactose. |
|
|
What is the function of the large intestine?
What do carbohydrates consist of & what is the general formula for them? |
*To absorb excess water from digestive secretions. *The undigested food is expelled as faeces.
*Carbohydrates contain carbon, hydrogen & oxygen. *(CH2O)n |
|
|
What are the 3 types of carbohydrates?
What are the uses of carbohydrates & which would be used? |
*Monosaccharides - Single sugars. *Disaccharides - Double sugar. *Polysaccharides - More than 2 sugars.
*Structural use - Cellulose, chitin. *Energy use - Glucose. *Storage use - Glycogen, starch. *Used in nucleic acids - Ribose, deoxyribose. |
|
|
What are monosaccharides?
Give examples of monosaccharides. |
*Simplest sugars which have the same number of Carbon & Oxygen atoms. *Monomer for starch, glycogen & cellulose.
*Glucose, Fructose & Galactose. *Glucose - C6H12O6 |
|
|
How are disaccharides formed & what do disaccharides form when they are hydrolysed?
Give examples of disaccharides. |
*Disaccharides hydrolyse to form Monosaccharides due to the hydrolysis of the glycosidic bonds. *Disaccharides form Polysaccharides. *Sucrose formed by condensation of glucose + fructose. Non reducing sugar. *Lactose formed by condensation of glucose + galactose. Reducing sugar. *Maltose is formed by condensation of 2 α - glucose molecules. Reducing sugar. |
|
|
Explain what polysaccharides are? |
*Polymers of monosaccharides. *Insoluble due to size & have no osmotic influence. *Do not diffuse easily. *Split into disaccharides & monosaccharides by hydrolysis. *Starch & cellulose are polysaccharides. |
|
|
Explain the structure of starch & cellulose? |
Starch:- *α - helical structure -> Good for storage, its compact. *Insoluble -> main plant storage sugar. Cellulose:- *Polymer of ß - glucose. *Each monomer is inverted. *Forms chains which run parallel with hydrogen bonds between the chains to form microfibrils which are strong. *Is structurally important in plant cell walls. |
|
|
Explain a biochemical test for reducing & non reducing sugars? |
Benedict's reagent:- *Add 2cm3 of food sample into a test tube. Grind up the sample into a liquid if not already. *Add the same volume of Benedict's reagent. *Heat mixture in a gently boiling water bath for 5 minutes. When a reducing sugar is present the solution will turn from a blue liquid to a red precipitate. |
|
|
What are proteins & what are proteins polymers of? |
*Determined by genetic codes. *Structure use - Collagen {bone, cartilage, tendon}, Keratin {hair}, Actin {muscle} *Enzymes - Amylase, Pepsin, Catalase. *Transport - Haemoglobin {oxygen}. *Active transport - Sodium - Patassium pumps in cell membranes. *Muscles {Myosin & Actin} *Hormones - Insulin, Glucagon. *Antibodies - Immunoglobulins. *Proteins are polymers of amino acids. |
|
|
What are amino acids? |
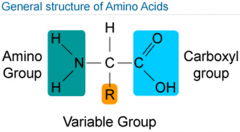
*Lots of proteins. *There are 20 naturally occuring amino acids. *Half are created in the body & the other half are consumed in diet. |
|
|
What is a peptide bond? |
*Peptide bond connects 2 amino acids which join together to form a dipeptide through a condensation reaction. *A hydrolysis reaction breaks peptide bonds. *This requires the use of a water molecule to reform the amino & carboxyl groups on each of the 2 new amino acids where the bond has split. |
|
|
What is a polypeptide & what are the 4 levels of protein structure? |
*Many amino acids make up a polypeptide chain. *Amino acid polymerisation for polypeptides is part of protein synthesis. *The sequence of amino acids in a chain is determined by the sequence of the genetic code in DNA. *Primary, secondary, tertiary & quarternary structures. |
|
|
Explain the primary & secondary structures of a protein. |
Primary structure:- *Refers to sequence of amino acids -> determines rest of protein structure. Secondary structure:- *Amino acid chain folds & take a particular shape. *Linked by hydrogen bonds. *Polypeptide chain becomes twisted into α - helix or ß - pleated sheet structure. |
|
|
Explain the tertiary & quarternary structures of a protein. |
Tertiary structure:- *3D shape *Hydrogen bonds - Numerous easily broken. *Ionic bonds - Quite strong. *Disulfide bonds - Strong covalent bonds. Quarternary structure:- *Final 3D structure with a number of individual polypeptide chains linked together. *May include non - protein {prosthetic} groups associated with molecules such as haem {iron} groups in haemoglobin. |
|
|
What are fibrous & globular proteins? |
Fibrous Proteins:- *Water insoluble, long & narrow proteins. *Many polypeptide chains run parallel, cross - linked with bonds which makes them stable & structurally strong. *E.g. Keratin, Collagen, Fibrin. Globular Proteins:- *More spherical & have specific shapes. *Small ones are soluble. *Often have complementary shape to another specific molecule. *E.g. Hormones, Enzymes, Haemoglobin & Antibodies. |
|
|
Explain the biochemical test for proteins. |
Biuret test:- *Place a sample of the solution to be tested in a test tube & add an equal volume of sodium hydroxide solution at room temperature. *Add a few drops of very dilute {0.05%} copper{II} sulfate solution & gently mix. *A purple colouration indicates the presence of peptide bonds, meaning there is a protein. |
|
|
What are enzymes? |
*Globular proteins, act as catalysts. *They lower the activation energy. *Active site is made up of a relatively small number of amino acids. *Substrate is the molecule that the enzyme breaks down. *Forms an enzyme - substrate complex. |
|
|
What is the lock & key model? |
*This model proposes that enzymes work in a lock & key method. *The substrate {key} will only fit into the active site of 1 particular enzyme {lock}. *Substrate has a complementary shape to the active site. *Limitation - is that an enzyme, like a lock, is considered to be rigid. But scientists observed other molecules could bind to enzymes at sites other than the active site. |
|
|
What is the induced fit model? |
*Proposes that the enzyme actually changes shape slightly to fit substrate. *Suggests enzymes are flexible & mould themselves around substrate. *When enzyme changes shape, strain is put on substrate. *This distorts particular bond & lowers activation energy needed to break bond. |
|
|
What factors affect the rate of reaction of enzyme action? |
*pH *Temperature *Substrate concentration *To measure enzyme - catalysed reactions, measure:- -> The formation of the products of the reaction. -> The disappearance of the substrate. |
|
|
How does pH affect enzyme action? |
*Each enzyme has an optimum pH at which it works best at. *A change in pH alters the charges on the amino acids that make the active site. *This affects the ionic bonds which means the substrate can no longer bind. *A change in pH can result in the enzyme becoming denatured. |
|
|
How does temperature affect enzyme action? |
*Each enzyme has an optimum temperature at which it works best at. *Rise in temperature increases kinetic energy of molecules which increases the number of collisions & increases the rate of reaction. *Increasing temperature vibrates molecules violently & breaks the hydrogen bonds which alters the 3D shape. *This causes the active site to no longer fit the substrate. *The enzyme denatures at 60C. |
|
|
How does substrate concentration affect enzyme action? |
*The rate of reaction increases as the substrate concentration increases, until the enzyme is working at full capacity. *This is due to collisions between the enzyme and the substrate. *In low substrate concentrations, not all enzymes are being used. *As the substrate concentration increases more enzymes molecules are used. *When all active sites are in use, substrate concentration does not affect rate of reaction. |
|
|
What is enzyme inhibition? |
*Inhibitors can slow down or stop catalytic activity of enzymes. *Inhibition is a natural process, a mechanism to switch enzymes on or off when needed. *Inhibition tends to be reversible as the enzyme returns to normal when the inhibitor is removed. *Reversible inhibitors can be competitive or non - competitive. |
|
|
What are competitive inhibitors? |
*They compete with substrate molecules to occupy the active site. *Have similar structure to substrate but can't convert into products. *Increase in concentration of substrate reduces effect of inhibitor. |
|
|
What are non - competitive inhibitors? |
*Bind to enzyme away from active site. *Decreases maximum rate of reaction. *Changes overall shape of enzyme. *Reduces amount of active enzymes. *Increase of substrate concentration has no effect on inhibitor. |

