![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
116 Cards in this Set
- Front
- Back
|
Define pool boiling.
|
(REPLACE)
|
|
|
Define forced convection boiling.
|
(REPLACE)
|
|
|
Define subcooled boiling.
|
(REPLACE)
|
|
|
Define saturated boiling.
|
(REPLACE)
|
|
|
Define excess temperature.
|
Delta T(ex) = T_s - T_sat
|
|
|
Define free convection boiling.
|
(REPLACE)
|
|
|
Define nucleate boiling.
|
(REPLACE)
|
|
|
Define film boiling.
|
(REPLACE)
|
|
|
Define transition boiling.
|
(REPLACE)
|
|
|
Define critical heat flux.
|
(REPLACE)
|
|
|
Define the Leidenfrost point.
|
(REPLACE)
|
|
|
Define homogenous condensation.
|
Cold vapor contacts vapor
|
|
|
Define direct contact condensation.
|
Cold liquid contacts vapor
|
|
|
Define surface condensation.
|
Cold solid contacts vapor
|
|
|
Define film condensation.
|
(REPLACE)
|
|
|
Define dropwise condensation.
|
(REPLACE)
|
|
|
Define the dimensionless parameter: Reynolds (Re)
|
Inertial Forces/Viscous Forces
|
|
|
Define the dimensionless parameter: Prandtl (Pr)
|
Kinematic Viscosity/Thermal Diffusivity
|
|
|
Define the dimensionless parameter: Jakob (Ja)
|
Phase change heat transfer
|
|
|
Define the dimensionless parameter: Bond (Bo)
|
Gravitational Forces
|
|
|
Sketch the boiling curve and identify key features and regimes.
|
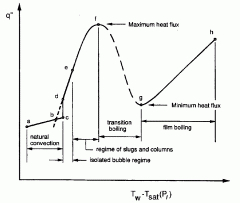
Look at the picture. It's pretty close.
|
|
|
Define the mean vapor mass fraction for internal forced convection boiling.
|
(REPLACE)
|
|
|
Explain any major safety considerations for the boiling or condensation process.
|
(REPLACE)
|
|
|
Define parallel flow.
|
(REPLACE)
|
|
|
Define counter flow.
|
(REPLACE)
|
|
|
Define cross flow.
|
(REPLACE)
|
|
|
Define single pass.
|
(REPLACE)
|
|
|
Define multi-pass.
|
(REPLACE)
|
|
|
Define fouling factor.
|
(REPLACE)
|
|
|
Define thermal resistance.
|
(REPLACE)
|
|
|
Define overall heat transfer coefficient.
|
U
|
|
|
Define overall surface efficiency.
|
(REPLACE)
|
|
|
Define log mean temperature difference.
|
(REPLACE)
|
|
|
Define minimum heat capacity rate.
|
(REPLACE)
|
|
|
Define maximum heat capacity rate.
|
(REPLACE)
|
|
|
Define heat capacity ratio.
|
(REPLACE)
|
|
|
Define effectiveness.
|
(REPLACE)
|
|
|
Define NTU.
|
(REPLACE)
|
|
|
Define mixed and unmixed flow in cross-flow heat exchangers.
|
(REPLACE)
|
|
|
Describe the configuration of the following types of heat exchangers: concentric tube, shell and tube, cross flow, and compact.
|
(REPLACE)
|
|
|
Define the overall heat transfer coefficient in terms of thermal resistances in series.
|
(REPLACE)
|
|
|
Explain the use of baffles in heat exchangers.
|
(REPLACE)
|
|
|
Explain when the outlet temperatures for the cold fluid can and cannot be higher than the outlet temperature for the hot fluid.
|
(REPLACE)
|
|
|
Explain how increasing the surface area for the heat exchange affects the heat transfer rate and the effectiveness.
|
(REPLACE)
|
|
|
Explain how increasing the overall heat transfer coefficient affects the heat transfer rate and the effectiveness.
|
(REPLACE)
|
|
|
Define relative volatility.
|
(REPLACE)
|
|
|
Define heavy key.
|
(REPLACE)
|
|
|
Define light key.
|
(REPLACE)
|
|
|
Define intensive and extensive variables.
|
(REPLACE)
|
|
|
Explain the Gibbs phase rule to determine the degrees of freedom for a system.
|
(REPLACE)
|
|
|
Define the equilibrium constant.
|
(REPLACE)
|
|
|
Define the bubble point and dew points for a binary vapor-liquid mixture using Raoult's Law or modified Raoult's Law.
|
(REPLACE)
|
|
|
Define and determine azeotropes for binary vapor-liquid mixtures.
|
(REPLACE)
|
|
|
Define liquid-liquid extraction.
|
(REPLACE)
|
|
|
Define solvent.
|
(REPLACE)
|
|
|
Define carrier.
|
(REPLACE)
|
|
|
Define solute.
|
(REPLACE)
|
|
|
Define Extraction factor.
|
(REPLACE)
|
|
|
Define partially miscible systems.
|
(REPLACE)
|
|
|
Define minimum solvent and maximum solvent flow rate.
|
(REPLACE)
|
|
|
Define slurry.
|
(REPLACE)
|
|
|
Define overflow.
|
(REPLACE)
|
|
|
Define underflow.
|
(REPLACE)
|
|
|
Define leaching stage.
|
(REPLACE)
|
|
|
Define constant solution underflow.
|
(REPLACE)
|
|
|
Define variable solution underflow.
|
(REPLACE)
|
|
|
List and explain the assumptions for an ideal leaching stage.
|
(REPLACE)
|
|
|
Identify different types of cascade configurations.
|
(REPLACE)
|
|
|
Define cascade and hybrid systems.
|
(REPLACE)
|
|
|
Define and identify sections of a cascade system.
|
(REPLACE)
|
|
|
Define the washing factor.
|
(REPLACE)
|
|
|
Define absorption and stripping factor.
|
(REPLACE)
|
|
|
Discuss different types of equipment for absorption and stripping processes.
|
(REPLACE)
|
|
|
Define three different tray types for tray towers. Discuss pros and cons of each type.
|
(REPLACE)
|
|
|
List and discuss design considerations for absorption or stripping processes.
|
(REPLACE)
|
|
|
Explain different ways to calculate efficiency of absorption and stripping towers. (Include overall efficiency and Murphree vapor efficiency)
|
(REPLACE)
|
|
|
Describe four ways to determine overall column efficiency.
|
(REPLACE)
|
|
|
Define HETP.
|
(REPLACE)
|
|
|
Define HTU.
|
(REPLACE)
|
|
|
Define H_OG.
|
(REPLACE)
|
|
|
Define NTU.
|
(REPLACE)
|
|
|
Define N_OG.
|
(REPLACE)
|
|
|
Describe the different possibilities for driving forces used in determining rate based separations found in packed columns.
|
(REPLACE)
|
|
|
Describe the local and overall mass transfer coefficients and how they are related to one another in rate based separation processes.
|
(REPLACE)
|
|
|
What are the basic assumptions included in the rate based separations method for packed columns?
|
(REPLACE)
|
|
|
How do the operating lines differ in concentrated absorption and stripping processes from the dilute systems we focused on for Exam 2?
|
(REPLACE)
|
|
|
Define distillation.
|
(REPLACE)
|
|
|
Define refulx ratio.
|
(REPLACE)
|
|
|
Define boilup ratio.
|
(REPLACE)
|
|
|
Draw and label a simple binary distillation column with a total condenser and a partial reboiler.
|
(Reference it yourself)
|
|
|
List at least three design considerations for a binary distillation system.
|
(REPLACE)
|
|
|
List and explain the assumptions made for constant molal overflow.
|
(REPLACE)
|
|
|
Sketch and label and example McCabe-Thiele diagram for binary distillation (Include operating lines and compositions where appropriate).
|
(REPLACE)
|
|
|
Define q thermal quality of the feed) and list the possible values for different feed conditions.
|
(REPLACE)
|
|
|
Describe how the McCabe-Thiele diagram is altered with the presence of multiple feed streams, side streams, open steam, or direct cooling.
|
(Refer to Test III)
|
|
|
Describe four methods for calculating overall column efficieny for a distillation column.
|
(REPLACE)
|
|
|
Describe how the feed stage is determined on a McCabe-Thiele diagram.
|
(REPLACE)
|
|
|
List at least two reasons why liquid-liquid extraction is preferred over distillation.
|
(REPLACE)
|
|
|
Describe four different types of equipment used in liquid-liquid extraction, includeding advantages and disadvantages of each type.
|
(REPLACE)
|
|
|
Explain at least three different design considerations for liquid-liquid extraction.
|
(REPLACE)
|
|
|
Describe the FUG method for multicomponent distillation.
|
F - Fenske determines N_min
U - Underwood determines R_min G - Gilliland uses N_min and R_min to determine N_actual and R_actual. |
|
|
Explain the assumptions made in the Fenske equation to approximate N_min.
|
(REPLACE)
|
|
|
Explain the best way to calculate the distribution of non-key components using the Fenske equation.
|
(REPLACE)
|
|
|
Explain the difference between Class 1 and Class 2 pinch points.
|
(REPLACE)
|
|
|
Explain the range of most used reflux rations and to which system they are commonly applied.
|
(REPLACE)
|
|
|
Describe the benefits of batch distillation versus continuous distillation.
|
(REPLACE)
|
|
|
Explain why the distillate product composition is calculated as an average.
|
(REPLACE)
|
|
|
Describe the two different modes of operation for batch distillation including how you would solve them graphically and which circumstances each is most applicable.
|
Constant Reflux -
Constant distillate composition - |
|
|
Define leachant.
|
(REPLACE)
|
|
|
Define leached solids.
|
(REPLACE)
|
|
|
Define slurry.
|
(REPLACE)
|
|
|
Define leaching.
|
(REPLACE)
|
|
|
Define washing.
|
(REPLACE)
|
|
|
Describe two types of solid-solvent contacting.
|
(REPLACE)
|
|
|
Describe three types of leaching processes and explain when each method is preferred.
|
Batch -
Semi-Batch - Continuous - |
|
|
Explain why separate washing stages are included in countercurrent leaching and washing process.
|
(REPLACE)
|

