![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
105 Cards in this Set
- Front
- Back
|
what is bacteria? |
bacteria is a very small living organism made of only one cell |
|
|
list the two ways bacteria are classified |
bacteria are classified by: 1. gram stain 2. oxygen requirements |
|
|
describe a gram stain: what is it? what is gram positive? what is gram negative? |
a gram stain designation refers to whether or not the bacterial cells respond to a gram stain
gram positive has a spongy layer with nothing to protect it so it soaks up the dye and turns blue or purple. the layer makes it harder for the drug to get through making gram + more virulent
gram negative is red or pink |
|
|
describe oxygen requirements (there are three types) |
1. aerobic = require oxygen 2. anerobic = cannot tolerate oxygen 3. facultative anaerobic= generally aerobes, can grow without oxygen |
|
|
what is the most common bacteria a patient can obtain from dental procedures? |
strep viralence |
|
|
define what antimicrobials are and what they do
what kind of drugs are included as antimicrobials?
list the three ways antimicrobials differ from each other |
antimicrobials are drugs that destroy, prevent the multiplication or growth, or prevent pathogenic action of a microbe. Antimicrobials can be natural or synthetic compounds; kill or suppress growth of microorganisms
antimicrobials include: antibacterial, antiviral, antifungal, etc.
- differ in physical, chemical, and pharmacological properties - differ in their mechanism of action - differ in antibacterial spectrum of activity |
|
|
define antibiotic |
an antibiotic is a natural compound made by microorganisms that have the ability to harm another microorganism |
|
|
what are the four types of antibiotic therapy? |
1. empiric 2. documented 3. de- escalation 4. prophylaxis |
|
|
antibiotic therapy: empiric |
antibiotic therapy: empiric
- presumptive because you do not definitively know what type of microorganism you're fighting
- base choice on likely organism on the premise of presentation and suspected site/source and bacteria |
|
|
antibiotic therapy: documented |
antibiotic therapy: documented
- culture results obtained and targeting definitive microorganism |
|
|
antibiotic therapy: de-escalation |
antibiotic therapy: de-escalation you started with a big lot of drugs with a broad spectrum or high dose then when you have more information then you can reduce and target your drug therapy
- once the definitive organism +/- evaluate patient status - peel off agents, decrease dose, decrease duration |
|
|
what should you consider when figuring out what bacteria a patient may have? |
has the patient had repeat visits and other cultures?
CF or chronic smokers have different bacteria than healthy patients or non smokers |
|
|
antibiotic therapy: prophylaxis |
antibiotic therapy: prophylaxis tries to prevent an infection for things you may be able to expect ie dental procedures, rheumatic fever |
|
|
what do you need to know when you treat with antibiotics? |
- know the microbiology - know the patient - know the drugs |
|
|
antimicrobial drug classification: define the four descriptors |
1. site and mechanism of action 2. chemical structure 3. spectrum of activity 4. how do they have their effect |
|
|
antimicrobial drug classification: 1. site and mechanism of action |
antimicrobial drug classification: site & mechanism of action
- inhibit cell wall vs. protein synthesis |
|
|
antimicrobial drug classification: 2. chemical structure |
antimicrobial drug classification: chemical structure
- penicillins vs. cephalosporins vs. macrolide - b-lactam vs. monolactam
|
|
|
antimicrobial drug classification: 3. spectrum of activity
|
antimicrobial drug classification: spectrum of activity - broad spectrum vs. narrow spectrum - gram positive vs gram negative agent - anti- MRSA, anti- pseuomonal
|
|
|
antimicrobial drug classification: 4. how they have their effect |
antimicrobial drug classification: how they have their effect bactericidal (kill bacteria) vs. bacteriostatic (inhibit growth aka stops replication) |
|
|
what is the primary determinant of an antimicrobial's clinical use? |
the primary determinant of an anti-microbial's clinical use is if it's narrow spectrum or broad spectrum |
|
|
narrow spectrum |
- active against a single species or limited group of pathogens - often used for documented therapy |
|
|
broad spectrum |
- active against a wide range of pathogens - often used for empiric therapy |
|
|
bacteriostatic vs. bactericidal |
if you left a bacteria alone then it would keep replicating because there's nothing to stop it
bacteriostatic drug stunts replication, drugs inhibit the growth of bacteria but do not kill them. need immunologic mechanisms to "kick in" to eliminate the organisms
bacteriostatic drugs can be used with an immunosuppressed patient that helps slow down the bug until the body's natural defenses can kick in
bactericidal: drugs kill organisms so that number of viable organisms decrease rapidly after exposure |
|
|
minimum inhibitory concentration (MIC) |
minimum inhibitory concentration (MIC) is the lowest concentration of a drug that prevents visible bacterial growth after 24 hours of incubation in a specified growth medium |
|
|
microbiology results and spectrum of activity: what information does it provide? (list three) |
microbio lab tests: 1. provide info about the in vitro activity of drugs vs. organism 2. reports organisms and susceptibilites to antimicrobials 3. based on MIC for the bug/ drug combo |
|
|
what can the three results be from the MIC bug/drug combo |
- sensitive: organism should be eradicated by treatment with the antibiotic at the recommended/ "usual" dosage - intermediate: organism may/may not be eradicated at the recommended / "usual" dosage dependent on achievable drug concentration and organism MIC - resistant: organism will not be inhibited by concentrations achievable by antibiotics at the recommended/ "usual" dosage |
|
|
Antimicrobial susceptibility methodologies: broth microdilution (BMD) |
there's a different organism in each row and a different concentration of drug in each of the columns - progressively decreasing levels of drug from left to right (no drug in furthest right column) - CLSI standard method; gold standard for MIC
|
|
|
describe the procedure for the broth microdilution (BMD) |
- 8 bacterial organism represented by each row, A-H, tested against a single antibiotic (entire plate) - each column of wells contains a standard antibiotic concentration that doubles when moving from left to right ie progressively decreasing levels of drug from left to right (no drug in the right column) - a small inoculum of bacteria is inoculated into each well - MIC is determined by the first well where there is no visible growth (clear liquid under transmitted light) - MIC of this antibiotic for organism (A = 4 mg/L, B- 8 mg/L, C= 2 mg/L, etc) |
|
|
MIC |
MIC determines drug therapy, S means sensitivity and it will work against organism just bc numbers are higher than others does not mean higher is more effective
susceptible, intermediate, resistant always stays the same for drug based on usual dose & formulation of the drug. drugs within the same class will yield different results MIC is the minimum serum concentration that you need to kill the bacteria |
|
|
pharmacodynamic parameters: concentration dependent |
- concentration- dependent: increased killing as the drug concentrations exceed the MIC - higher peak concentrations > better killing - peak/ MIC or AUC/MIC ratios are important |
|
|
pharmacodynamic parameters, what are the two parameters? |
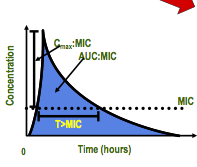
1. concentration- dependent (y axis) 2. time- dependent (x- axis) |
|
|
pharmacodynamic parameters: time- dependent |
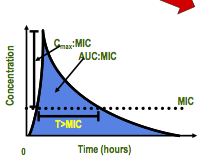
- kill bacteria when the drug concentrations exceeded the MIC - duration of time serum concentration > MIC - penicillins, cephalosporins |
|
|
t/f: different patients can have different MICs than these b/c your e coli may need a different concentration of drug to kill it than someone else's e. coli |
true |
|
|
if a drug is said to be only "concentration dependent" what does that mean? |
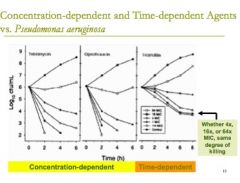
a concentration dependent drug means that you just have to get the drug above the MIC but length of dosing interval doesn't matter
if you give more of the drug, then the bacteria will be killed faster |
|
|
if a drug is said to be a "time- dependent kill" what does that mean? |
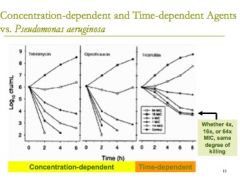
a drug that is a time- dependent kill then the same concentration of drug needs to be able to stay above the MIC for a pre-prescribed portion of that dosing interval to have the best possible killing effect
even if you add a lot more of the drug, it doesn't matter because you just have to wait for it to work |
|
|
pharmacodynamic properties of cabapenems |
for carbapenems, time above MIC (T> MIC) is the variable linked with the ability to stop the growth of an organism or to kill it |
|
|
what factors impact the probability of achieving the dynamic goal? |
clinicians manipulate the drugs and use them in the combo with other agents to beat the bugs when they come back with I or R in their reports
- distribution of organism MIC for the drug - population distribution of the pharmacokinetic parameters for the drug - drug protein binidng |
|
|
% of dosing interval required for the drug concentrations that exceed MIC of the pathogen for beta lactam antibiotics (% time > MIC)
drug class, stasis end point, max kill end point carbapenems pencillins cephalosporins |
drug class, stasis end point, max kill end point carbapenems 20, 40 pencillins 30, 50 cephalosporins 40, 60-70 |
|
|
simulated plasma concentration- time curves for variable dosing schema |
- often clinicians give a drug more slowly so they can maintain the drug concentration for a longer amount of time
for example, bacteria with MIC of 4 give 500 mg dose over an hour and give it all of once then the MIC drops below 4 quickly. instead, give 500 mg over 4 hours
if you double the dose with the same bug, you still cross the line of MIC around the same time
doubling the dose only buys you a little time but giving the same dose over a longer period of time buys you more time |
|
|
what do clinicians do with drugs that have short half lives? |
clinicians use a combination of drugs and manipulate how we give the short half life drugs that are affected are usually seen with zosyn, cephapine, mirpenem |
|
|
what is a major factor limiting antibiotic use? |
resistance, the concentration of drug does not exceed the MIC adequately (not for long enough or at all)
bacteria have evolved various mechanisms to either destroy or limit the effectiveness of antibiotics |
|
|
resistance: what are 2 types of resistance? list and define them. |
1. intrinsic: bacteria is born with resistance, like gram negative and they have an outer wall that makes it more difficult to treat. "lack of activity before antibiotic exposure". 2. acquired: develops during therapy due to mutations or transferred from one organism to another |
|
|
resistance: what are the 3 main mechanisms? |
1. inactivation of drug by microbial enzymes 2. decreased accumulation of the drug (increased efflux or decreased uptake) 3. reduced affinity by the target for the drug (whatever drug is supported to bind) |
|
|
resistance: 1. inactivation of drug by microbial enzymes |
bugs will produce enzymes that will chew up antibiotics (PCN are betalactams, bugs produce betalactamase) |
|
|
resistance: 2. decreased accumulation of the drug |
decreased accumulation of drug at organism itself so drug cannot get in because of conformational change in outer layer, prin change, or mutated transport mechanism or there's an efflux pump, gets in then gets out |
|
|
resistance: 3. reduced affinity by the target for the drug |
- if the binding site mutates then the drug cannot mind - alteration in the target where the drug is intended to work |
|
|
what should you keep in mind about pharmacokinetics?
what factors affect selection? |
remember ADME pharmacokinetics factors affecting selection: - oral bioavailablity: is it good or not? - IV: "standard" vs prolonged infusion - serum concentrations (~ surrogate for tissue) - distribution to site infection - route of elimination (how is it metabolized? ie does it need to be renally adjusted?) - half life - local antibiogram, local resistance patterns
|
|
|
antibiogram |
collect the first 200 samples of psuedomonas that come into lab, report the susceptibility patterns |
|
|
what are three categories to keep in mind for the antimicrobial selection process? |
1. host factors 2. medication considerations 3. route of administration |
|
|
antimicrobial selection process: 1. host factors |
antimicrobial selection process: host factors - age - weight - organ function (renal/hepatic) - site of infection - common causative organisms - prior infectious history - comorbidites - pregnancy - genetic/ metabolic abnormalities - allergies/ ADR's |
|
|
antimicrobial selection process: 2. medication considerations |
antimicrobial selection process: medication considerations - pharmacokinetics (ADME) - pharmacodynamics - tissue penetration - cost - drug levels required |
|
|
antimicrobial selection process: 3. route of administration |
antimicrobial selection process: route of administration
- bioavailablity (F) - severity of infection - location of infection - organ function (eg GI) |
|
|
general statements, antimicrobial therapy: site of infection |
site of infection is a VERY important factor when you're considering antimicrobial selection - defines the most likely organisms, esp helpful during empiric antimicrobial selection - determines choice, dose and route
|
|
|
general statements, antimicrobial therapy: site of infection
how do you determine efficacy of selection (ie what kind of questions should you be thinking about)? |
efficacy is determined by concentrations of drug above the MIC at the site of infection - does the antibiotic get there? - are there sufficient concentrations? - distribution |
|
|
what should you think about about how a drug is distributed? are there areas where it's harder for drugs to get to? |
- difference exist in plasma vs. tissue concentrations - areas of compromised penetrations: CSF fluid, bone, prostate, eye, abscesses
for example, urinary tract infections are often easier to treat than other sites because drugs concentrate in the urine .
CSF patients- as the patient's infection goes away with meningitis it becomes harder to get drugs in (inflammation from infection allows drugs in more easily)
|
|
|
general statements, antimicrobial therapy: drug interactions |
- oral contraceptives - warfarin |
|
|
general statements, antimicrobial therapy: drug interactions, oral contraceptives |
oral contraceptives: normal flora in the GI tract are responsible for some of the synthesis of your hormones. If you take drugs that disturb normal balance you will disturb the way hormones are made
tell patients to have a backup method of birth control for the entire cycle, not just during the antibiotics |
|
|
general statements, antimicrobial therapy: drug interactions, warfarin |
warfarin: normal flora disrupts the synthesis of vitamin K so it will mess up the balance of K
need INR around 3 days into antibiotic therapy and need to re- titrate the warfarin when done with antibiotics |
|
|
general statements, antimicrobial therapy: elimination |
think about how the drug is eliminated: renal vs. liver |
|
|
general statements, antimicrobial therapy: dosing |
- loading doses (aminoglycosides, 1st dose in renal patient) - dose adjustments for renal (hepatic) the loading dose will always be a normal first dose then the maintenance doses will be adjusted for renal impairment
- therapeutic drug monitoring required/useful (esp for aminoglycosides) |
|
|
general statements, antimicrobial therapy: dosing, dose adjustments for renal (hepatic) |
- time dependent vs. concentration dependent - hemodialysis, CRRT (continuous renal replacement therapy), ECMO (extracorporeal membrane oxygenation, external machine that oxygenates the lungs) |
|
|
general statements, antimicrobial therapy: pregnancy/ lacation |
consider if the medication crosses |
|
|
general statements, antimicrobial therapy: CNS penetration |
consider the status of the meninges, size/lipophilicity of molecule
- if you increase the lipid penetration then the drug is more likely to get into CNS |
|
|
considerations, concomitant drug therapy: combination antibiotic therapy (3 considerations) |
1. used frequently in treatment of infections 2. understand interaction potential 3. influences the selection of appropriate drug therapy, dosage, necessary monitoring |
|
|
considerations, concomitant drug therapy: combination antibiotic therapy treatment of infections |
- to achieve broader spectrum of activity (especially empiric therapy) - to achieve better killing of bacteria (synergy) - to minimize development of resistance ie dead bugs do not multiply or develop resistance |
|
|
considerations, concomitant drug therapy: combination antibiotic therapy understand the interaction potential |
- pharmacokinetic vs. pharmacodynamic interactions - general rule: try to avoid using 2 agents with the same mechanism of action
|
|
|
considerations: cultures, when do you do cultures?
where do you culture? |
do cultures before first antibiotic dose!
you culture from the infected site! |
|
|
considerations: cultures, where do you culture patients with:
pneumonia non-intubated intubated |
considerations: cultures, where do you culture patients with: pneumonia
non- intubated: sputum intubated: BAL/ET aspirate
mini- BAL for all |
|
|
considerations: cultures, where do you culture patients with:
decubitus ulcers diabetic foot infections suspected necrotizing fasciitis |
debrided tissue from decubitius ulcers that look clinically infected, diabetic foot infections, suspected necrotizing fasciitis
if you have an ulcer, debride it and get culture from stuff below what you debrided do not swab necrotic tissue because you risk mistreating the patient with the wrong antibiotic so you want to ensure you have the correct sample |
|
|
considerations: cultures, where do you culture patients with:
meningismus |
cerebrospinal fluid from patients with meningismus |
|
|
considerations: cultures, where do you culture patients with:
abscesses |
pus from aspiration or drainage of abscesses |
|
|
considerations: cultures, where do you culture patients with:
endocarditis |
additional blood cultures for suspected endocarditis (at least three blood cultures for native valve endocarditis, four or five for prosthetic valve endocarditis)
endocarditis has a bad yield so make sure to send extra blood bottles. the cause could be gone when you draw the blood or the infection could be encapsulated. |
|
|
considerations: cultures, blood cultures (3)
|
1. avoid contamination of the blood culture media by normal skin flora during the process of collection 2. single sets are not enough for suspected bacteremia or candidemia 3. two blood cultures done at least 10 mins apart should be drawn and a culture from the infected site should be taken prior to the first dose of antibiotics |
|
|
considerations: cultures, blood culture
how do you avoid contamination of blood culture media by normal skin flora? |
- effective venipuncture disinfection - avoiding blood culture collection through existing IV lines |
|
|
considerations: cultures, blood culture
how do you get optimal yield for suspected bacteremia or candidemia? |
optimal yield is obtained with three for four sets of blood culture to capture suspected bacteremia or candidemia |
|
|
considerations: cultures, blood culture
what's the best practice for drawing blood cultures?
is there any time component? the culture should be taken before ________? describe the specific practice to draw the culture. |
- two blood cultures should drawn at least 10 minutes apart - a culture from the infected site should be taken prior to the first dose of antibiotics
- catheter tip + 3 (4) peripheral cultures - cultures for strict anaerobes should be sent on anaerobic transport media
if you're going to send a tip you need to send blood cultures that are NOT FROM THAT TIP |
|
|
considerations: cultures when should swab cultures be done? |
swab cultures should only be done on: - bodily fluids - tissue - pus - exudates
|
|
|
general principles of/approach to antimicrobial therapy (7) |
1. evaluate infectious/ non-infectious causes of fevers 2. have a high suspicion for infection and possible or likely source 3. obtain appropriate cultures 4. select presumptive therapy 5. verify presence of an infection from a specific site 6. narrow spectrum of coverage 7. monitor efficacy/toxicity of regimen |
|
|
what do you consider when selecting presumptive therapy? |
when selecting presumptive therapy, base your selection on: - likely organism(s) - host factors - medication factors |
|
|
treatment: risk, toxicity |
all antimicrobials carry a risk (and a cost) to the patient
direct toxicity may be encountered (eg ototoxicity due to aminoglycosides, lipodystrophy due to HIV protease inhibitors), and hepatotoxicity due to anti- tuberculosis agents such as isoniazid and rifampin |
|
|
treatment: risk, allergic reactions |
all antimicrobials carry a risk (and a cost) to the patient
allergic reactions are common and can be serious |
|
|
treatment: risk, superinfection |
all antimicrobials carry a risk (and a cost) to the patient
superinfection sometimes follows the eradication of the normal flora and colonization by a resistant organism
|
|
|
treatment: risk, infectious disease |
all antimicrobials carry a risk (and a cost) to the patient
infectious disease therapy should be directed toward as narrow a spectrum of infectious agents as possible |
|
|
T/F: you want the treatment to be specific for the pathogen and result in as little alteration as possible for the host's microflora |
True. |
|
|
T/F: for antibiotics that possess concentration- depending killing properties, you should optimize the time above the MIC |
false- with doses of antibiotic over MIC |
|
|
which of the following should be considered when choosing an antimicrobial: A. site of infection B. allergies C. spectrum of activity D. Only A & C E. All of the above |
E |
|
|
antimicrobial targets: DNA synthesis (1) DNA gyrase (1) |
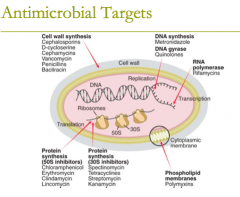
antimicrobial targets: DNA synthesis: metronidazole DNA gyrase: quinolones, (fluoroquinolones) |
|
|
antimicrobial targets: RNA polymerase (2) |
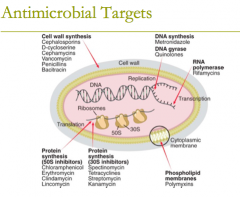
antimicrobial targets: RNA polymerase: 1. rifamycins 2. Rifampin |
|
|
antimicrobial targets: protein synthesis (8) |
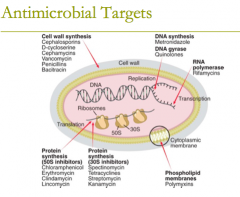
antimicrobial targets: protein synthesis (50S inhibitors) G MASCCOT (what the letters spell in case it helps to remember)
1. chloramphenicol 2. tetracyclines 3. glycylcyline (tigecycline) 4. macrolides 5. clindamycin 6. streptogramins (quinupristin/dalfopristin) 7. oxazolidinones (linezolid) 8. aminoglycosides |
|
|
antimicrobial targets: phospholipid membranes (1) |
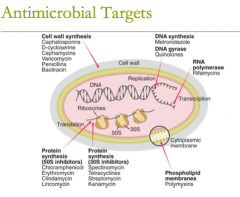
antimicrobial targets: phospholipid membranes (1)
attacks the cytoplasmic membrane 1. polymyxins |
|
|
antimicrobial targets: folate synthesis inhibitors (2) |
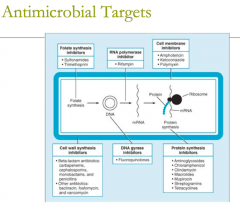
antimicrobial targets: folate synthesis inhibitors (2) 1. sulfonamids 2. trimethoprim |
|
|
antimicrobial targets: cell membrane inhibitors (3) |
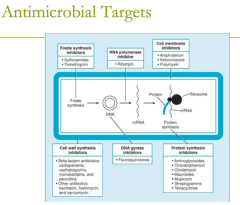
1. Amphotericin 2. Ketoconazole 3. Polymyxin |
|
|
antimicrobial targets:
cell wall synthesis inhibitors (8) what are the most important ones? (two classes and five subclasses) |
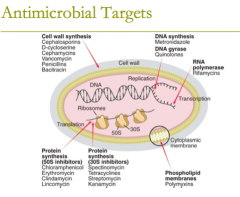
antimicrobial targets: cell wall synthesis
1. bacitracin 2. beta-lactam antibiotics: ie carbapenems, cephlosporins, etc 3. cephamycins 4. D- cycloserine 5. fosfomycin 6. monobactams (aztreonam) 7. Penicillins 8. Glycopeptide- Vancomycin |
|
|
what are the three major phases of cell wall synthesis? |
drugs will work at 1, 2, or 3 phases to prevent wall form forming on new organisms 1. monomer synthesis 2. monomer polymerization 3. cross- linking of polymers |
|
|
three major phases of cell wall synthesis: monomer synthesis |
three major phases of cell wall synthesis: monomer synthesis - synthesis of peptidoglycan monomers from amino acids and sugars - "precursor formation" - takes place in cytoplasm |
|
|
three major phases of cell wall synthesis: monomer polymerization |
three major phases of cell wall synthesis: monomer polymerization - linkage to form a long polymer - lipid- mediated and takes place at the cystoplasmic membrane |
|
|
three major phases of cell wall synthesis: cross-linking of polymers |
three major phases of cell wall synthesis: cross-linking of polymers - mediated by enzymes called transpeptidases, including penicllin- binding proteins (PBPs) extracellular takes place in periplasmic space |
|
|
describe the three parts of the beta- lactam structure
how do you make PCN?
|
beta- lactam structure: 1. has a thiazolidine ring connected to a beta lactam ring to which is attached a side chain 2. nucleus is structural requirement for main biologic activity 3. side chain determines many of the antibacterial and phamacologic characteristics
add side chains >> get PCN
|
|
|
beta-lactams: mechanism of action |
beta lactams interfere with the last step of bacterial cell wall synthesis (transpeptidation or cross- linkage)
- inactivate penicillin- binding proteins (PBPs) by binding to them - inhibition of transpeptidase (PBPs) leads to autolysin-mediated autolysis and cell death |
|
|
are beta lactams time dependent or concentration dependent? are beta lactams bactericidal or bacteriostatic? |
beta lactams are time-dependent beta lactams are bactericidal against actively dividing bacteria |
|
|
beta lactams: mechanisms of resistance |
- beta lactamase enzyme which hydrolyzes the beta lactam ring - decreased permeability of the drug through the outer cell membrane - altered PBPs with lower affinity for beta lactam antibiotics - efflux of drug across the outer membrane of gram-negative organisms
refresher: hydrolyze = break, renders drug inactive |
|
|
natural pencillins list parenteral and oral
describe their spectrum of activity what are they hydrolyzed by? |
parenteral: PCN G oral: PCN VK
spectrum of activity: - active against sensitive strains of gram positive cocci (streptococcus sp.), anerobes (Clostridium perfringes), and select gram negative cocci (Neisseria sp) - spirochetes
hydrolyzed by penicillinase (beta- lactamase) |
|
|
PCN G (parenteral) 3 forms |
- potassium or sodium salt (parenteral) - procaine (IM): comparable leves to IV for 24 hours - benzathine (IM): low levels for long period of time (weeks) |
|
|
penicillinase-resistant PCN: list the antistaphylococcal penicillins |
- methicillin (not available) - nafcillin, oxacillin (parenteral) - cloxacillin, dicloxacillin (PO) |
|
|
penicillinase-resistant PCN: spectrum of activity clinical uses |
penicillinase-resistant PCN spectrum of activity: penicillinase- producing staphylococci (Methicillin-susceptible S. aureus (MSSA) - PCN- susceptible streptococcus sp - NOT active against MRSA, enterococci, listeria spp
clinical uses: infections caused by MSSA skin/soft tissue infectios, endocarditis, osteomyelitis, etc |

