![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
What is an antibody? |
Antibody or immunoglobulin is a specialized glycoprotein, produced from activated B cells (plasma cells) in response to an antigen |
|
|
Ig constitutes ____% of total serum proteins |
20-25% |
|
|
Electrophoresis of human serum proteins |
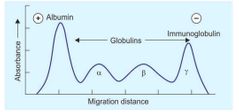
|
|
|
General structure of antibody |
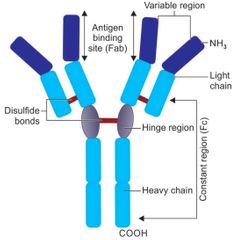
|
|
|
H chains |
γ, α, µ, δ and ε |
|
|
In humans, __ of the light chains are kappa and ___are lambda type (ratio 3:2) |
60% 40% |
|
|
hypervariable regions or complementarity determining regions (CDRs) |
Within the variable region, there are some zones (hot spots) that show relatively higher variability in the amino acid sequences There are three hot spots in the L and four in the H chain, respectively |
|
|
Features of hinge region |
This region is rich in proline and cysteine. The hinge region is flexible. The hinge region is sensitive to various enzymatic digestions. |
|
|
Enzymatic digestion of immunoglobulin |
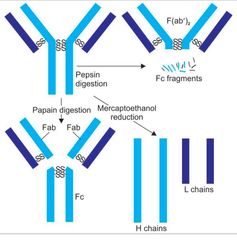
|
|
|
the binding of an antibody to its antigen does not result in any direct biological effect. Rather, variety of secondary “effector functions” are produced These effector functions include: |
1.Fixation of complement 2.Binding to various cell types Phagocytes, lymphocytes, platelets, mast cells, NK cell, eosinophils and basophils placental trophoblasts(IgG) |
|
|
IgG constitutes about ____ of total Ig in the body. |
70-80% |
|
|
What is the half life of IgG and how many subclasses does it have? |
23 days 4 subclasses |
|
|
complement-fixing ability of subclasses IgG1 , IgG2 , IgG3 , IgG4 |
IgG3 > IgG1 > IgG2 IgG does not fix complements |
|
|
Which subclass of IgG is involved in phagocytosis? |
IgG1 and IgG3 bind to Fc receptors present on phagocytes (macrophages, neutrophils) with high affinity and enhance the phagocytosis (opsonization) of antigen bound to them. IgG2 has an extremely low affinity for Fc receptors of phagocytes |
|
|
Why does IgG play a major role in neutralization of toxins ? |
as it can easily diffuse into extravascular space |
|
|
Which reactions does IgG mediate & what does it represent? |
•Precipitation & neutralization •Chronic or past illnes(raised after a long time following infection) |
|
|
How does IgG mediate coagglutination? |
IgG subclasses (except IgG3) mediate coagglutination reaction by binding to protein-A of S. aureus |
|
|
Where is IgM present? |
present only in intravascular compartment, not in body fluids or secretions |
|
|
In how many forms IgM exists? |
1.Monomeric ( When present as membrane-bound antibody on B cells) 2.Pentameric (When present in secreted form) |
|
|
Overall functions of IgG |
1. Complement fixing 2. Phagocytosis 3. Coagglutination |
|
|
Functions of IgM |
1.Acute infection (first Ig to be produced following an infection) 2.complement fixing (most potent activator of classical complement pathway-5 Fc regions) 3.serves as B cell receptor for antigen binding 4.opsonin( IgM>IgG - 500 to 1000times) 5. Fetal immunity 6. Protection against intravascular organisms 7.Mediates agglutination (IgM>IgG - 20 times) |
|
|
What is to the % of IgA in total serum & In what r the forms in which it exists? |
10 - 15% •Monomeric (IgA in serum) • dimeric ( secretory IgA) - J chain , secretory component |
|
|
Functions of serum IgA |
initiates various functions such as •antibody-dependent cell-mediated cytotoxicity (ADCC), •degranulation of immune cells etc. |
|
|
Where is secretory IgA predominantly found? |
body secretions like milk, saliva, tears, intestinal and respiratory tract mucosal secretions |
|
|
Functions of IgA |
mediates local or mucosal immunity effective against bacteria like Salmonella, Vibrio, Neisseria & viruses like polio and influenza |
|
|
Where r these synthesized Dimeric secretory IgA J chain Secretory component |
Dimeric s.IgA & J chain - plasm cells situated near mucosal epithelium Secretory component - mucosal epi.cells |
|
|
Function of secretory component of IgA |
1. Helps IgA cross epithelial surface & reach lumen 2. protects IgA from denaturation by bacterial proteases produced by intestinal flora. |
|
|
Subclasses of IgA |
1. IgA1 ( serum) 2. IgA2 ( secretions) |
|
|
Features of IgE |
1. Has Lowest serum conc 2.shortest half life & daily production 3. Only heat labile Ig 4. Homocytotrophism 5. Mainly extravascular in distribution |
|
|
Functions of IgE |
1. Mediates type I hypersensitivity rxns (by binding to the mast cells causing degranulation.) Seen in allergies 2. Elevated in helminthic infections ( Coats on the surface of eosinophils ADCC) |
|
|
IgD |

|
|
|
On what basis Ig molecules are divided into isotypes, idiotypes and allotypes? |
Based on the location of antigenic determinants |
|
|
Antigenic determinants of immunoglobulin |
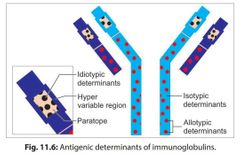
|
|
|
How do isotypes vary from each other? |
in the amino acid sequences of the constant region of their heavy chains. |
|
|
What are idiotopes and idiotypes? |
The unique amino acid sequence present in paratope region (in VH and VL regions) of one member of a species acts as antigenic determinant to other members of the same species. Such antigenic determinants are called as idiotopes and the sum total of idiotopes on an Ig molecule constitutes its idiotypes |
|
|
Does somatic hypermutations in idiotypes evoke AI response |
They may act as foreign to the host itself but are present in small numbers .So do not evoke AI |
|
|
What are allotypes immunoglobulins |
The antigenic determinants present in the isotype genes in the constant region of H and L chains, encoded by multiple alleles are called as allotypes. |
|
|
When are abnormal immunoglobulins like Bence jones proteins produced? |
produced in a neoplastic condition of plasma cells called multiple myeloma / light chain disease which are accumulated in patient’s serum and excreted in urine •coagulation at 50° & redissolving at 70° |
|
|
What are the other abnormal immunoglobulins? |
1.Waldenstrom’s Macroglobulinemia (B cell lymphoma - excess IgM) 2.Heavy chain disease(exssve heavy ch) 3.Cryoglobulinemia( seen in Multiple mye & hep C infection) |
|
|
What are monoclonal antibodies? |
The antibodies derived from a single clone of plasma cell; all having the same antigen specificity, i.e. produced against a single epitope of an antigen. |
|
|
How are monoclonal antibodies produced & who developed it? |
Monoclonal antibodies are produced by Hybridoma technique, developed by G Kohler and C Milstein |
|
|
When are polyclonal & monoclonal antibodies produced in serum? |
•When an antigen having multiple epitopes enters the body -POLY •when only one clone of B cell is stimulated by a single epitope of an antigen - MONOCLONAL |
|
|
What is the principle of hybridoma technique? |
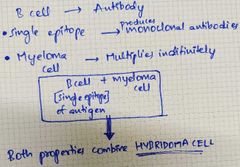
|
|
|
Hybridoma technology |
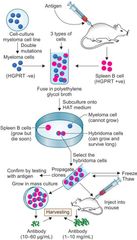
|
|
|
Steps of hybridoma technique |
1.Mouse splenic Bcell 2.Myeloma cells(double mutated) 3.Fusion(3 types generated) 4.purification 5.HAT medium(aminopterin×de novo) •hypoxanthine, aminopterin,thymidine -salvage pw(2 enzymesHGPRT&TKinase) -myeloma lacks HGPRT 6.Fate of three types of cells on HAT media 7.Selection of individual hybridoma cells -diluted; selected by RIA/ELISA 8.Maintainance of mAb(cultured-pure) (Ascitic fluid -mixed with other Ab) •purifican- chromatography |
|
|
What is the concentration of hybridom cells produced in pure form & mixed form |
Pure form- 10-60 ug/ml Mixed form - 1-10mg/ml |
|
|
What happens if we inject mouse derived mAb into humans? |
the mouse proteins being foreign; can induce immune response in humans producing human anti-mouse antibodies (HAMA) |
|
|
Types of mAb |
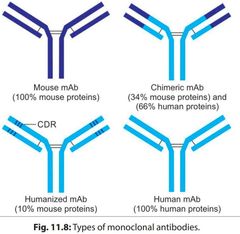
|
|
|
What are the postulates which explain the mechanism of antibody diversity? |
1. Multiple chromosomes 2. Multiple genes exist for each segment |

