![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
47 Cards in this Set
- Front
- Back
|
What is pain? |
Pain: An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage; it is what a patient says it is!
A cerebral construct, pain is a perception usually but not always associated with tissue-damaging stimuli (nociceptive information) |
|
|
What are the somatosensory receptors? |

|
|
|
How is pain sensed via nociceptors? |
Membranes of nociceptors have receptor proteins that allow response to tissue-damaging stimuli: signal transduction. Temp - TRPV1,2,3, TREK1. Acid - TRPV1, ASIC, DRASIC. Force/sting - MDEG, DRASIC, TREK-1. Cold - TRPM8. -> DRG |
|
|
What are the pain fibres? |
Adelta - thinly myelinated, 1.5um, 5-35m/s - sharp pricking FAST pain - thermal/mechanical C - unmyelinated, .2-1.5 diameter, .5-2m/s - SLOW burning pain |
|
|
How are sensory peripheral fibres classified? |

|
|
|
What is the effect of Ad pain sensation? |
Precise localisation of insult Reflex withdrawal |
|
|
What is the effect of C fibre pain sensation? |
Autonomic effects: vasoactive, inflammatory, neurogenic info, healing. |
|
|
What are the 2 types of C fibres? |
1. peptidergic: release peptides peripherally e.g. substance P/CGRP 2. Peptide poor: distinct receptors - P2X3 ATP receptors + projections |
|
|
What genetic defects prevent pain sensation? |
NaV1.7 - sodium channel subunit congenital insensitivity to pain CIPA (trkA - NGF receptor mutation) congenital insensitivity to pain with anhydrosis |
|
|
What is the cause of prolonged pain? |
Inflammation - sensitisation of area of injury: hyperalgesia/allodynia - enables protection + facilitates healing. |
|
|
What are the 4 cardinal signs of inflammation? |
Calor (heat) Rubor (redness) Dolor (pain) Tumor (swelling) |
|
|
What are hyperalgesia, allodynia and spontaneous pain? |
Hyperalgesia - increased pain in response to painful stimulus Allodynia - pain in response to innocuous stimulus, change in perception Spontaneous - absence of stimulus. |
|
|
What are the effects of burns and pressure pain to further pain perception? |
Burns: primary thermal hyperalgesia increased sensitivity at SITE Pressure: Increased sensitivity to pressure pain: both at site and adjoining i.e. primary and secondary mechanical hyperalgesia + allodynia |
|
|
What happens to pain sensation upon tissue injury/ prolonged noxious stimulation? |
Peripheral sensitisation - reduction in activation threshold, increase in responsiveness of peripheral ends of nociceptors. Occurs via upregulation inflamm mediators - mast cell histamine, neuropeptide release: SP/CGRP |
|
|
What is the effect of inflammatory mediators on peripheral sensitisation? |
Stimulate nociceptive fibre endings and prolong activity. Modification and increased expression ion channels and receptors via intracellular messenger cascades i.e. phosphorylation and transcriptional activation. TTXr Na channels change threshold for opening + kinetics, K+ close, TRPV1 increase sensitivity to heat. Some nociceptors tonically active. |
|
|
Describe prostaglandin synthesis and the effects on peripheral nerves. |
Phospholipase --PLA2--> arachidonic acid --COX1&2--> PGs. COX1 present at low levels all the time, COX2 only in inflammation. PGs sensitise nerves by increasing number of other receptors and increasing number open Na channels. |
|
|
How do steroids inhibit inflammation? |
Inhibit PLA2 |
|
|
What occurs upon prolonged nociceptor input to the dorsal horn? |
Central sensitisation - activity dependent increase in excitability of dorsal horn neurones (outlasts stimulus) -> modified responsiveness - low threshold mechanoreceptor AB fibre input produce response -> pain allodynia, hyperalgesia |
|
|
What are triggers of central sensitisastion? |
Nociceptor afferents release GLUT and peptides. Depolarisation relieves block on NMDA GLUT receptors. |
|
|
What immune cells contribute to peripheral amplification? |
Mast cells Macrophages Neutrophils |
|
|
What examples of clinically relevant stimuli cause pain amplification? |
Tissue trauma, surgery, joint inflammation in RA |
|
|
What is chronic pain? |
Pain > 12wks - associated underlying condition |
|
|
What is abnormal/bad pain? |
Abdnormal activity in nerve/CNS i.e. NEUROPATHIC PAIN - dysfunction/lesion PNS/CNS DYSFUNCTIONAL PAIN - no known neural lesion/ongoing peripheral inflamm - triggered non nociceptive inputs/spontaneous. Causes - stroke, infection (postherpetic neuralgia), drug treatments, diabetes, chronic alcoholism. |
|
|
Describe the phenomenon of referred pain. i.e. CONVERGENCE |
Pain from internal organs is difficult for the brain to comprehend as it has no body schema or ‘map’ of the interior of the body to project the pain on to. Normally the pain is felt in the region of the body surface that has its afferent input in the same spinal segment (dermatome) as that where the active visceral pain fibres terminate. |
|
|
Describe the path of pain from noxious stimulus to brain. |
Free nerve endings of slow C/fast Ad fibres -> afferent nerve fibre -> DRG -> synpase to lateral ST tract in SC -> reticular formation in midpons -> synapse in thalamus -> SS cortex + limbic system. |
|
|
What other insults stimulate C fibres? |
Mechano, thermo, chemo + crude/gentle touch |
|
|
What is the composition of the DRG? |
C mechanoheat nociceptors 40% C mechano nociceptors 20% AM nociceptors 12% SA 12% RA 10% D hair 6% |
|
|
What are silent nociceptors? |
Population of skin C fibres that become active in inflammation to then be sensitised to inocuous stimuli. |
|
|
What spinal fibre levels do pain afferents synapse in? |
Ad - I C/Ad peptidergic - I-IIo C non peptidergic - II Ad hair follicle afferents - IIi - III Ab hair follicle and tactile - IIi - V |
|
|
Where do spinothalamic tract cells arise from? |
Lamina I, IV + V. |
|
|
What is the neospinothalamic tract? FIRST DISCRIMINATIVE PAIN Neospinothalamic tract: signals acute (fast) pain and localisation of pain so that remedial action can be taken (eg remove limb from damage) |
Projection neurones in lamina IV/V innvervated by Ad fibres + convergent input C/Ab fibres. - wide dynamic range cells. ANTERIOR STT -> lateral thalamus - VPL/VPM -> 1oSS cortex, VPI - 2oSS, Central lateral -> anterior cingulate cortex, emotion + prefrontal cortex + striatum - cognitive function + strategy. |
|
|
What is the paleospinothalamic tract? SECOND PUNISHING PAIN Paleospinothalamic tract: signals slow pain; activates emotional and autonomic aspects of pain. |
LATERAL - projection neurones in lamina 1 - mainly C fibres directly + indirectly from interneurons in lamina II + cells with input from Ad - nociceptive specific cells. -> medial posterior thalamus. MDvc nucleus, posterior thalamus - medial subnucleus POm + ventral medial nucleus posterior VMpo. Few fibres to ventral posterior. MDvc -> ACC. POm, Vmpo -> rostral insula |
|
|
What functions do the ACC, 1oSS, PFC, Insula and amygdala have in pain? |
ACC - emotional, motivation/aversive learning 1oSS - somatosensory discrimination PFC - cognition, evaluation Insula - pain map, integration, emotion Amygdala - emotion/memory |
|
|
What are the targets of neoSTT and paleoSTT? |
Neo - 1o + 2o SSC Paleo - anterior cingulate C + rostral insular C |
|
|
What are other non-perceptual elements of nociception? |
Spinoreticular - reticular formation: arousal, autonomic, behavioural reflex arcs, to PAG - descending pain modulation and autonomic/motor output Spinomesencephalic - to parabrachial nucleus - lamina I to amygdala - limbic activation and visceral integration |
|
|
What are 4 physiological mechanisms of pain? |
1. Transduction - noxious stimuli translated to electrical activity at sensory nerve endings 2. Transmission - propogation of impulses along pain pathways 3. Perception - affective/motivation 4. Modulation - Stages 1-3 modified |
|
|
How can pain be modulated at different levels of the pain pathway? |
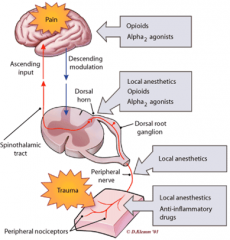
|
|
|
What is Melzack and Wall's revised gate control theory of pain? |
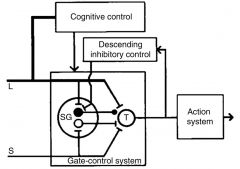
Black circle inhibitory and white circle excitatory links from substantia gelatinosa to the transmission cells in dorsal horn of SC |
|
|
Why can tissue damage sometimes not result in pain? |
Tissue damage does not always result in pain because the nociceptor input can be ‘gated off’ in the dorsal horn by innocuous stimuli & descending pathways from the brainstem |
|
|
How does the gate theory present on a neuronal scale? |
Ad + C fibres synapse on Lamina I + lamina IV/V cells releasing GLUT + excitatory peptide substance P.
Inhibitory interneurones in lamina II activated by non-pain afferents and also descending aminergic fibres from the brain stem inhibiting terminals and lamina I cels by releasing enkephalins (peptides) + GABA |
|
|
Where are opiate/enkephalin receptors located? |
Primary afferents + lamina I dendrites. If afferents destroyed enkephalin receptors lost + opiates mostly ineffective |
|
|
How do centrally acting analgesics e.g. opiates/ clonidine work? |
By mimicking the actions of lamina II (substantia gelatinosa) dorsal horn cells.
Opiates (morphine, codeine, heroin) are agonists for μ (mu) enkephalin receptors on lamina I cells and C fibre afferents. They block the excitatory action of the C fibres on the lamina I cells.
Somework by mimicking descending amine pathways that switch on lamina II cells-- noradrenaline a2 agonists e.g. clonidine |
|
|
What is the analgesic basis of transcutaneous electrical nerve stimulation (TENS) and acupuncture? |
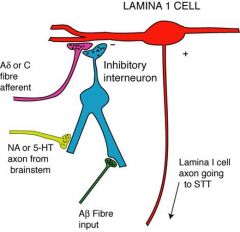
stimulation of non-noxious/mildly stimulate lamina II cels which inhibit lamina II cells. |
|
|
What is the PAG? |
Region around cerebral aqueduct rich in enkephalin containing neurones. Electrical stimulation produces powerful analgesia. Region activates locus coeruleus and raphe nucleus of rostal ventromedial medulla and controls descending inhibition of pain. Receives input from hypothalamus and other limbic regions. |
|
|
What do the superior parietal cortex SPC-insula-amygdala and ACC-PFC-PAG pathway moderate? |
SPC-insula-amygdala - cognitive attention control of intensity. ACC-PFC-PAG - emotion/placebo control of unpleasantness - sensitive to opiate antagonists. |
|
|
Where is central nociceptor termination located? |
C fibres inner are lamina I projection neurones and inter neurones in lamina II.
Ad fibres inner are laminae I and V projection neurones
Lamina V projection neurones also receive AB input and C fibre input.
Axons of projection neurones decussates close to where the nociceptors enter the spinal cord and form the anterolateral system/ST tract |
|
|
What are the two distinct aspects of the experience of pain? |

Back (Definition) |

