![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
57 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Bronchial walls of lungs are made up of three types of tissue
|
- Epithelial lining (exocrine gland cells for secretion of mucous and ciliated cells for movement of foreign objects.
- Smooth muscle for expansion and contraction - Connective tissue to provide structure |
|
|
|
Alveoli
|
Where most gas exchange takes place in lungs. Surfactant is secreted to decrease surface tension to allow alveoli to expand more readily. Premature infants lack surfactant.
|
|
|
|
Pulmonary circulation
|
Facilitates gas exchange. Less pressure and resistance than systemic circulation (MAP 18 vs 90)
|
|
|
|
Chemoreceptors associated with breathing.
|
Chemoreceptors detect the levels of carbon dioxide in the blood. To do this, they monitor the concentration of hydrogen ions in the blood, which decreases the pH of the blood. This is a direct consequence of an increase in carbon dioxide concentration, because carbon dioxide becomes carbonic acid in an aqueous environment.
The response is that the inspiratory centre (in the medulla), sends nervous impulses to the external intercostal muscles and the diaphragm, via the intercostal nerve and the phrenic nerve, respectively, to increase breathing rate and the volume of the lungs during inhalation. Chemoreceptors which affect breathing rate are broken down into two categories. central chemoreceptors are located on the ventrolateral surface of medulla oblongata and detect changes in pH of cerebrospinal fluid. They do not respond to a drop in oxygen, and eventually desensitize. They respond quickly. peripheral chemoreceptors: Aortic body detects changes in blood oxygen and carbon dioxide, but not pH, while carotid body detects all three. They do not desensitize. Their effect on breathing rate is less than that of the central chemoreceptors. |
|
|
|
Carbonic acid, bicarbonate, plasma PH, partial pressure of CO2.
|
Carbonic acid plays a very important role as a buffer in mammalian blood. When CO2 enters the blood from various cells, it is combined with water to produce carbonic acid. It then has a H+ taken away from it to become bicarbonate (HCO3-). In order to transport the bicarbonate that is in the blood stream out of the body, it enters another red blood cell, has H+ attached to it to form carbonic acid once again, then has H2O taken away from it and is expelled from the red blood cell as CO2. Then the carbon dioxide is permitted to be expelled out of capillaries and into the lungs.
The equilibrium between carbon dioxide and carbonic acid is very important for controlling the acidity of body fluids, and almost all living organisms have an enzyme, carbonic anhydrase, which catalyzes the conversion between the two compounds, increasing the reaction rate by a factor of nearly a billion |
|
|
|
Dyspnea
|
shortness of breath (SOB) is perceived to be difficulty of breathing or painful breathing. It is a common symptom of numerous medical disorders
|
|
|
|
Orthopnea
|
dyspnea which occurs when lying flat, causing the person to have to sleep propped up in bed or sitting in a chair. It is the opposite of platypnoea.
Orthopnoea is a symptom of heart failure. It can also occur in those with asthma and chronic bronchitis, as well as those with sleep apnea or panic disorder. The condition is often due to left ventricular failure and/or pulmonary edema. It is also associated with Polycystic Liver Disease. It is commonly measured according to the number of pillows needed to prop the patient up to enable breathing (Example: "3 pillow orthopnea"). |
|
|
|
PND
|
It is defined as sudden, severe shortness of breath at night that awakens a person from sleep, often with coughing and wheezing. It is most closely associated with congestive heart failure. PND commonly occurs several hours after a person with heart failure has fallen asleep. PND is often relieved by sitting upright, but not as quickly as simple orthopnea. Also unlike orthopnea, it does not develop immediately upon lying down.
|
Paroxysmal Nocturnal Dyspnea
|
|
|
Kussmaul breathing
|
Kussmaul breathing is the very deep and labored breathing with normal or reduced frequency, found among people with severe acidosis; it is a form of hyperventilation. The cause of Kussmaul breathing is respiratory compensation for a metabolic acidosis, most commonly occurring in diabetics in diabetic ketoacidosis. Blood gases on a patient with Kussmaul breathing will show a low pCO2 in conjunction with low bicarbonate because of a forced increased respiration (blowing off the carbon dioxide). The patient feels an urge to breathe deeply, an "air hunger", and it appears almost involuntary.
A metabolic acidosis soon produces hyperventilation, but at first it will tend to be rapid and relatively shallow. Kussmaul breathing develops as the acidosis grows more severe. Indeed, Kussmaul originally identified this type of breathing as a sign of coma and imminent death in diabetic patients. |
|
|
|
Pursed lipped breathing
|
Common in emphysema as it increases the resistance to exhalation, and therefore keeps the lungs from collapsing.
|
|
|
|
Cheyne-Stokes respiration
|
Cheyne-Stokes respiration (also known as periodic breathing) is an abnormal pattern of breathing characterized by oscillation of ventilation between apnea and hyperpnea, to compensate for changing serum partial pressures of oxygen and carbon dioxide. This abnormal pattern of breathing can be seen in patients with strokes, traumatic brain injuries, brain tumors, and congestive heart failure. In some instances, it can occur in otherwise normal people during sleep at high altitudes. It can occur in all forms of toxic metabolic encephalopathy. It is a symptom of carbon monoxide poisoning, along with syncope or coma. This type of respiration is also often seen after morphine administration.
Hospice personnel often note the presence of Cheyne-Stokes breathing as a patient nears death, and report that patients able to speak after such episodes do not report any distress associated with the breathing, although it is sometimes disturbing to the family. |
|
|
|
Hypoxia vs hypoxemia
|
Hypoxia is insufficient oxygen reaching cells due to hypoxemia, insufficient hemoglobin, arterial obstruction. Hypoxemia is insufficient oxygen content of arterial blood due to respiratory problems such as inadequate ventilation, or impaired diffusion across the alveolocapillary membrane.
|
|
|
|
Blood flow through the heart
|
R Atrium (much thinner than other parts, more easily damaged)
Tricuspid Valve R Ventricle Pulmonic Valve L Atrium Mitral Valve L Ventricle (most muscular part of heart) Aortic Valve |
|
|
|
Structure of heart
|
Pericardium
Myocardium (heart muscle) Endocardium (smooth epithelial tissue) |
|
|
|
Beta adrenergic control of heart
|
Beta-1 mostly in heart (conduction system) generally increases HR, strength of contraction. NE binds more with Beta-1
Beta-2 found mostly in bronchi and also in coronary arterioles - leads to dilation of both, Epinephrine binds mostly with Beta-2. Beta stim will inc HR and dilate bronchi, Beta block will reduce HR, constrict bronchi (caution for asthma pts) |
|
|
|
Alpha adrenergic control of heart
|
Some receptors in heart, mostly in vessels. Stimulation causes constriction.
|
|
|
|
Autonomic control of heart
|
Sympathetic (NE) generally inc HR and contraction
Parasympathetic - vagus nerve (CN-X) ACh, decrease rate. |
|
|
|
Types of antihypertensives
|
Beta Blockers (propranolol)blocks beta1&2 receptors, orthostatic hypotension, exacerbates asthma.
ACE Inhibitors (captopril, enalapril) interferes with Angiotensin Converting Enzyme - prev. conv AT1 to AT2, bronchospasm, hypotension, bradycardia. Calcium Channel Blockers (nifedipine, diltiazam), inhib Ca++ uptake, reflex tachycardia, hypotension |
|
|
|
Congestive Heart Failure
|
LVF is most common. S/S of left failure is dyspnea, pulmonary congestion. S/S of right failure is peripheral edema, vein distention
|
|
|
|
Functions of kidney
|
1 Excretion of waste products
2 Homeostasis 2.1 Acid-base balance 2.2 Blood pressure 2.3 Plasma volume 3 Hormone secretion |
|
|
|
Kidney excretion of waste products
|
The kidneys excrete a variety of waste products produced by metabolism, including the nitrogenous wastes: urea (from protein catabolism) and uric acid (from nucleic acid metabolism) and water.
|
|
|
|
Homeostatic functions of the kidney
|
The kidney is one of the major organs involved in whole-body homeostasis. Among its homeostatic functions are acid-base balance, regulation of electrolyte concentrations, control of blood volume, and regulation of blood pressure. The kidneys accomplish these homeostatic functions independently and through coordination with other organs, particularly those of the endocrine system. The kidney communicates with these organs through hormones secreted into the bloodstream.
|
|
|
|
Acid-base balance by kidneys
|
The kidneys regulate the pH of blood by adjusting H+ ion levels, referred as augmentation of mineral ion concentration, as well as water composition of the blood.
|
|
|
|
Blood pressure regulation by kidneys
|
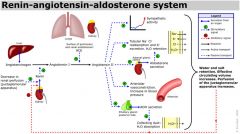
Sodium ions are controlled in a homeostatic process involving aldosterone which increases sodium ion reabsorption in the distal convoluted tubules.
|
|
|
|
Hormone secretion by kidneys
|
The kidneys secrete a variety of hormones, including erythropoietin, urodilatin, renin and vitamin D. Also responsible for gluconeogenesis, creating glucose from amino acids.
|
|
|
|
Glomerulus
|
Tuft of capillaries contained in Bowman's capsule. Main site of filtration. All components of blood are filtered except blood cells and very large proteins. In biological terms, ultrafiltration occurs at the barrier between the blood and the filtrate in the renal corpuscle or Bowman's capsule in the kidneys. The Bowman's capsule contains a dense capillary network called the glomerulus. Blood flows into these capillaries through a wide afferent arteriole and leaves through a narrower efferent arteriole. The blood pressure inside these capillaries is high because:
The renal artery contains blood at very high pressure which enters the glomerulus via the short afferent arteriole. The efferent arteriole has a smaller diameter than the afferent arteriole. The high pressure forces small molecules such as water, glucose, amino acids, sodium chloride and urea through the filter, from the blood in the glomerular capsule across the basement membrane of the Bowman's capsule and into the nephron. This type of high pressure filtration is ultrafiltration. The fluid formed in this way is called glomerular filtrate. |
|
|
|
Kidney filtration
|
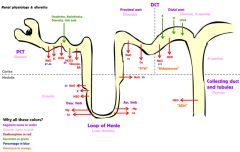
The blood is filtered by nephrons, the functional units of the kidney. Each nephron begins in a renal corpuscle, which is composed of a glomerulus enclosed in a Bowman's capsule. Cells, proteins, and other large molecules are filtered out of the glomerulus by a process of ultrafiltration, leaving an ultrafiltrate that resembles plasma (except that the ultrafiltrate has negligible plasma proteins) to enter Bowman's space. The ultrafiltrate is passed through, in turn, the proximal tubule, the loop of Henle, the distal convoluted tubule, and a series of collecting ducts to form urine.
|
|
|
|
nephron
|
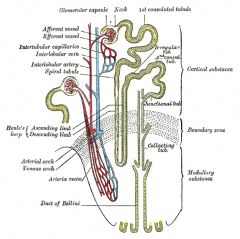
The basic structural and functional unit of the kidney. Its chief function is to regulate the concentration of water and soluble substances like sodium salts by filtering the blood, reabsorbing what is needed and excreting the rest as urine. A nephron eliminates wastes from the body, regulates blood volume and pressure, controls levels of electrolytes and metabolites, and regulates blood pH. Its functions are vital to life and are regulated by the endocrine system by hormones such as antidiuretic hormone, aldosterone, and parathyroid hormone. Each nephron is composed of an initial filtering component (the "renal corpuscle") and a tubule specialized for reabsorption and secretion (the "renal tubule"). The renal corpuscle filters out large solutes from the blood, delivering water and small solutes to the renal tubule for modification.
|
|
|
|
proximal tubule of the kidney
|
The proximal tubule as a part of the nephron can be divided into an initial convoluted portion and a following straight (descending) portion. Fluid in the filtrate entering the proximal convoluted tubule is reabsorbed into the peritubular capillaries, including approximately two-thirds of the filtered salt and water and all filtered organic solutes (primarily glucose and amino acids).
|
|
|
|
Loop of Henle
|
The loop of Henle (sometimes known as the nephron loop) is a tube, it is often u-shaped in diagrams for simplicity but in reality it looks more like one loop of a coil (hence, 'loop'). It extends from the proximal tube and it consists of a descending limb and ascending limb. It begins in the cortex, receiving filtrate from the proximal convoluted tubule, extends into the medulla, and then returns to the cortex to empty into the distal convoluted tubule. Its primary role is to concentrate the salt in the interstitium, the tissue surrounding the loop.
Its descending limb is permeable to water but completely impermeable to salt, and thus only indirectly contributes to the concentration of the interstitium. As the filtrate descends deeper into the hypertonic interstitium of the renal medulla, water flows freely out of the descending limb by osmosis until the tonicity of the filtrate and interstitium equilibrate. Longer descending limbs allow more time for water to flow out of the filtrate, so longer limbs make the filtrate more hypertonic than shorter limbs. Unlike the descending limb, the ascending limb of Henle's loop is impermeable to water, a critical feature of the countercurrent exchange mechanism employed by the loop. The ascending limb actively pumps sodium out of the filtrate, generating the hypertonic interstitium that drives countercurrent exchange. In passing through the ascending limb, the filtrate grows hypotonic since it has lost much of its sodium content. This hypotonic filtrate is passed to the distal convoluted tubule in the renal cortex. |
|
|
|
distal tubule of the kidney
|
The distal convoluted tubule is not similar to the proximal convoluted tubule in structure and function. Cells lining the tubule have numerous mitochondria to produce enough energy (ATP) for active transport to take place. Much of the ion transport taking place in the distal convoluted tubule is regulated by the endocrine system. In the presence of parathyroid hormone, the distal convoluted tubule reabsorbs more calcium and excretes more phosphate. When aldosterone is present, more sodium is reabsorbed and more potassium excreted. Atrial natriuretic peptide causes the distal convoluted tubule to excrete more sodium. In addition, the tubule also secernates hydrogen and ammonium to regulate pH.
|
|
|
|
components of renal blood blow
|
- Autoregulation to maintain constant GFR.
- Neural regulation (SNS) - Renin AII system - Atrial natriuretic peptide |
|
|
|
Stimulants of Renin - AII system
|
- Reduced BP
- Decreased Na conc. in distal tubule - SNS stimulation |
|
|
|
Concentration/dilution of urine
|
Antidiuretic hormone controls the final concentration of the urine. It is secreted from the posterior pituitary. It increases the water permeability of the distal tubule and the collecting ducts.
|
|
|
|
Acid-base balance in renal function.
|
Distal tubule regulates acid-base balance, secretes hydrogen into the tubule, reabsorbs bicarbonate. Buffers in the tubular fluid combine with hydrogen ion, allowing more hydrogen to be excreted.
Phosphate and ammonia are important renal buffers. |
|
|
|
Renal function and aging
|
Renal blood flow decreases over time. There is also a loss of nephrons, particularly between 40 and 80. Therefore there is a decreased ability to concentrate urine. Reabsorption of glucose, bicarbonate and sodium is less efficient. Less efficiency in excreting drugs.
|
|
|
|
gastrin
|
a hormone that stimulates secretion of gastric acid (HCl) by the parietal cells of the stomach, as well as aiding in gastric motility.
|
|
|
|
motilin
|
a polypeptide hormone secreted by M cells of the small intestine that increases the migrating myoelectric complex component of gastrointestinal motility and stimulates the production of pepsin.
|
|
|
|
secretin
|
a peptide hormone. Its primary effect is to regulate the pH of the duodenal contents via the control of gastric acid secretion and buffering with bicarbonate
|
|
|
|
Gastric acid secretion is stimulated by:
|
Acetylcholine
Gastrin Histamine (note, Tagamet and others are antihistamine) |
|
|
|
Gastric acid secretion is inhibited by:
|
Somatostatin
Prostaglandins Secretin Gastric Inhibitory Peptide |
|
|
|
Somatostatin
|
Somatostatin (also known as growth hormone inhibiting hormone (GHIH) or somatotropin release-inhibiting factor (SRIF)) is a peptide hormone that regulates the endocrine system and affects neurotransmission and cell proliferation via interaction with G-protein-coupled somatostatin receptors and inhibition of the release of numerous secondary hormones. Somatostatin is classified as an inhibitory hormone, whose actions are spread to different parts of the body. Somatostatin is secreted in several locations in the digestive system. Somatostatin is also produced by neuroendocrine neurons of the periventricular nucleus of the hypothalamus. These neurons project to the median eminence, where somatostatin is released from neurosecretory nerve endings into the hypothalamo-hypophysial portal circulation. These blood vessels carry somatostatin to the anterior pituitary gland, where somatostatin inhibits the secretion of growth hormone from somatotrope cells. The somatostatin neurons in the periventricular nucleus mediate negative feedback effects of growth hormone on its own release; the somatostatin neurons respond to high circulating concentrations of growth hormone.
|
|
|
|
Liver: functions
|
It plays a major role in metabolism and has a number of functions in the body, including glycogen storage, decomposition of red blood cells, plasma protein synthesis, and detoxification. It produces bile, an alkaline compound which aids in digestion, via the emulsification of lipids.
|
|
|
|
Portal vein
|
Vein from other organs (spleen, pancreas, stomach, small intestine, and large intestine), so that the liver can process the nutrients and by-products of food digestion.
|
|
|
|
Glycogen storage
|
Removal of sugar from blood by liver and conversion to glycogen, which is stored in the liver.
|
|
|
|
Hepatic detoxification
|
Alters structure of substances to decrease toxicity or increase elimination. Ammonia is converted to urea. Kupffer cells phagocytize worn our/dying blood cells, bacteria and other debris.
|
|
|
|
Hepatic synthesis
|
Many blood proteins are synthesized in the liver:
Albumins (protein) Fibrinogen Globulins Heparin Clotting factor |
|
|
|
bilirubin
|
Byproduct of RBC destruction. Aged blood cells are phagocytized in Kupffer cells. Hemoglobin is broken down into heme and globin. Heme is further broken down into iron (which is recycled) and bilirubin. Unconjugated bilirubin is lipid soluble and present in plasma. Conjugated bilirubin is water soluble and excreted in bile.
|
|
|
|
regulation of hormone release
|
- endocrine glands
- hormones from other endocrine glands - chemical factors - neural factors - feedback mechanisms |
|
|
|
water soluble hormone transport (insulin, parathyroid, hypothalamic, pituitary)
|
- circulate in unbound forms which allows them to be exposed to circulating enzymes quickly when released.
- half lives are shore (seconds to minutes) - pass through pores in the walls of capillaries |
|
|
|
lipid soluble hormone transport (e.g. thyroid, steroid)
|
- transport in blood bound to proteins.
- levels of free and bound hormone are usually equal so shifts in binding proteins will change the levels of free hormones. Lipid soluble are usually small in size compared to water soluble, making transport easier. |
|
|
|
TRH, thyrotropin releasing hormone
|
TRH is produced by the hypothalamus, near the paraventricular nucleus. It is released into the hypothalamic-pituitary portal system and circulates to the anterior pituitary (hypophysis). It stimulates the release of thyroid-stimulating hormone and prolactin.
|
|
|
|
TSH, thyroid stimulating hormone
|
Stored in the anterior pituitary and released in response to TRH, stimulates thyroid gland to release stored thyroid hormones (T3 and T4). Also increases the rate of iodine uptake and oxidation. Increases thyroid hormone synthesis.
|
|
|
|
Thyroid hormone actions
|
Increases rate of carbohydrate, fat and protein metabolism. Also essential for the effectiveness of growth hormone on normal growth and development.
|
|
|
|
TH synthesis
|
Uptake of iodide into thyroid cell, where it is oxidized to form iodine. Iodine is combined with tyrosine to form iodotyrosine. Coupling of one monoiodotyrosine with one diiodotyrosine forms triiodotyrosine (T3). Coupling two diiodotyrosines creates T4.
|
|
|
|
T3 and T4
|
Thyroid creates 10% T3 and 90% T4. In the body, T4 is converted to T3 (which is metab. effective). 99% of T3 and T4 are bound to thyroxine binding globulin for transport.
|
|
|
|
adrenal gland, structure and function
|
outer cortex - glucocorticoids and sex hormones
inner medulla - catecholamines. adult gland secretes 85% EPI and 15% NE activated during stress response. |
|

