![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
Avogadro constant
|
number of particles in a mole
|
|
|
Relative atomic mas (RAM)
|
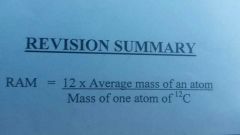
|
|
|
number of moles =
|
⚫mass (g) ÷ RMM
⚫volume (dm3) × concentration (mol dm-3) |
|
|
Def : mole
|
The mole is the amount of substance the contains as many entities as there are atoms in 12g of carbon 12.
|
|
|
ideal GAS equation
|
pV = nRT
p (Pa) V (m3) |
|
|
Relative Molecular Mass
|
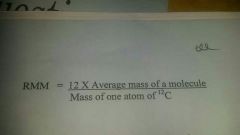
|
|
|
%Atom economy
|
(Mass of desired product ÷ sum of MR of all reactants) ×100
|
|
|
Def: Empirical formula
|
simplest ratio of the atoms of the elements in a compound.
|
|
|
Def: Molecular formula
|
actual number of atoms of the elements in a molecule. (whole number × empirical formula)
|

